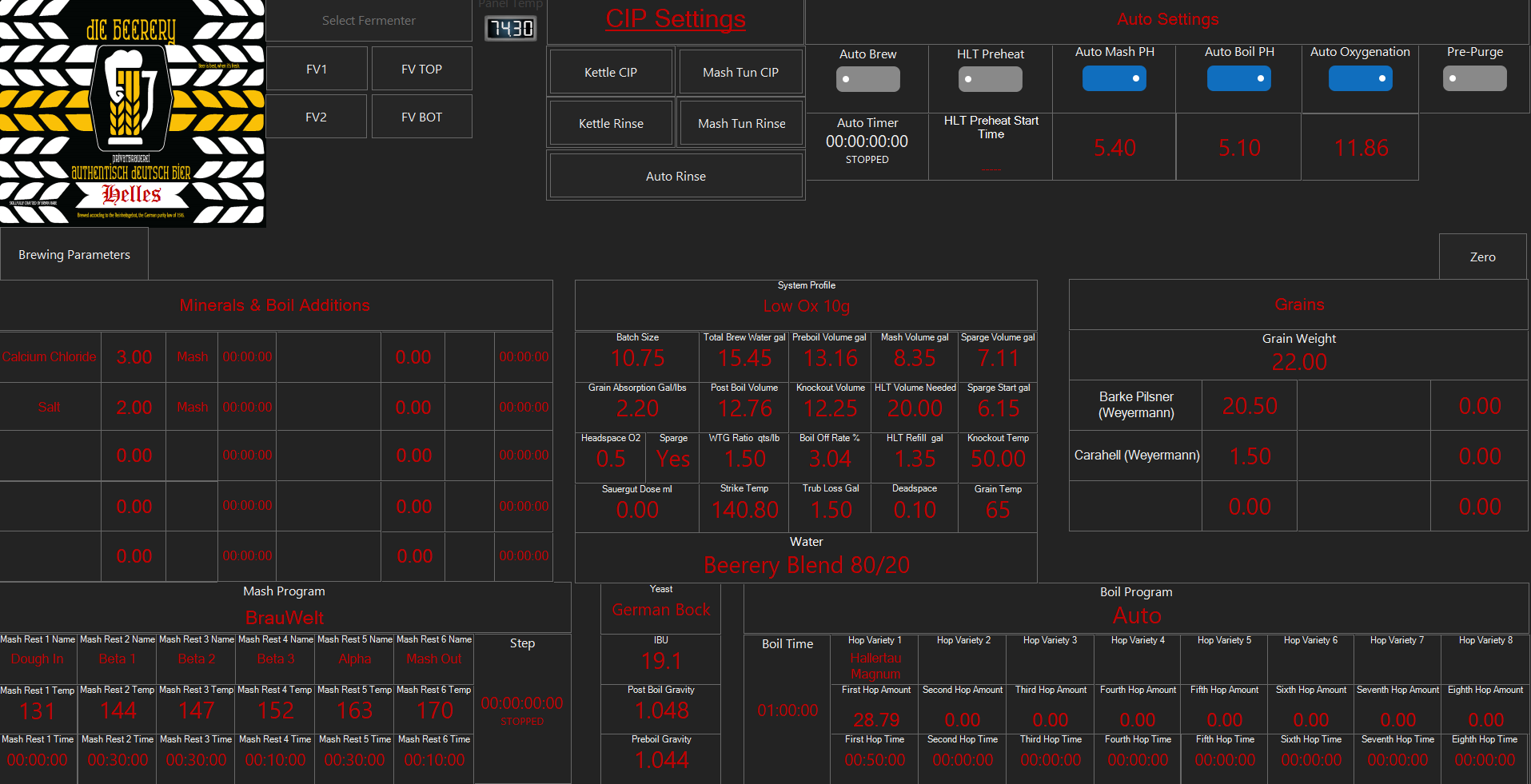
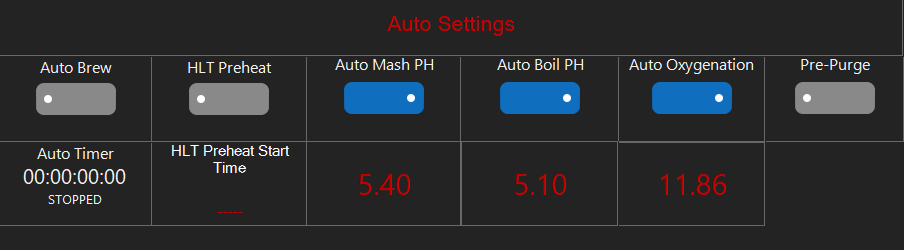
Larry, it saddens me to read of your predicament and having followed your postings for some time. I wish to continue doing so for much time to come and hope your health holds strong for all that time and more. However, on the topic under discussion here, my opinion is not in line with yours.
From my outset in dealings with professional brewers I found hardly any with a grasp of pH, not that I claim to be an expert. Similarly I came across few professional chemists who understood brewing beyond the odd snippet. Most brewers, however, knew when taking a gravity or pH reading to put the sample jar in running water until it had cooled.
To stay in business in the past breweries employed chemists to maintain beer quality, while today more likely to employ a graphics artist to design labels and can art and I would suppose the average head brewer knows any more now than then.
By observation you would seem to have been reading the archives of The Journal of the Institute of Brewing, so might I recommend another?
There, in the earliest days of what today we designate "pH", when that science was in the remit of the chemist and not the brewer, a reference to the then standard temperature of 18C (~65F). On page 411 are some mash readings which, 98 years later, are those expected today with a cooled sample. So did brewers take mash pH measurements out of the hands of the chemists?
I started brewing when pH meters were powered by mains electricity and housed in a laboratory, not handheld with a probe that would reach several feet, passed the sparge arm and into the grain in a mash tun. Then I would use pH strips which, by the time they were in a position to compare with the color chart were cool and if they read pH 5.8 it was certain there was too much alkalinity present in the liquor.
Just my version.
From my outset in dealings with professional brewers I found hardly any with a grasp of pH, not that I claim to be an expert. Similarly I came across few professional chemists who understood brewing beyond the odd snippet. Most brewers, however, knew when taking a gravity or pH reading to put the sample jar in running water until it had cooled.
To stay in business in the past breweries employed chemists to maintain beer quality, while today more likely to employ a graphics artist to design labels and can art and I would suppose the average head brewer knows any more now than then.
By observation you would seem to have been reading the archives of The Journal of the Institute of Brewing, so might I recommend another?
There, in the earliest days of what today we designate "pH", when that science was in the remit of the chemist and not the brewer, a reference to the then standard temperature of 18C (~65F). On page 411 are some mash readings which, 98 years later, are those expected today with a cooled sample. So did brewers take mash pH measurements out of the hands of the chemists?
I started brewing when pH meters were powered by mains electricity and housed in a laboratory, not handheld with a probe that would reach several feet, passed the sparge arm and into the grain in a mash tun. Then I would use pH strips which, by the time they were in a position to compare with the color chart were cool and if they read pH 5.8 it was certain there was too much alkalinity present in the liquor.
Just my version.