My mistake. You're correct. (Fixed my post)
I'm was confusing saturation vs what would be normal tap water values.
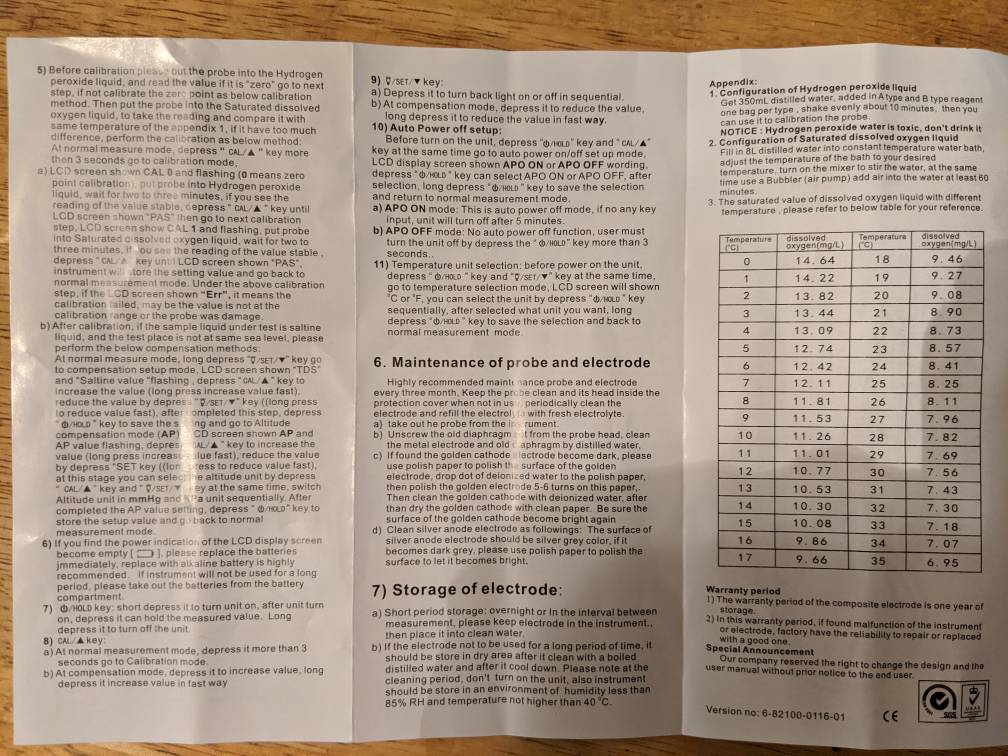
Supposedly at 21C the saturation limit is 8.9 per my meter Instructions posted above. Doing so with a stir plate or an aquarium bubbling stone.
This also depends on altitude and barometric pressure. I think the altitude is an initial calibration set point.
I guess my point is recognizing the higher DO and detecting the change in points. Setting the max and min your environment. I'm more concerned about the zero point accuracy vs the saturation. I liked seeing both close to what is expected.
Bear with me while I ask a highly heretical question that goes against the grain of our deeply held orthodoxy of brewing. Please don't dismiss or disparage just because "that's not the way it's done, so shut up and fall in line." It's a serious question that I can't answer, so please indulge me with a reasonable answer:
Why do we even bother to oxygenate the wort?
After all, we take great steps to eliminate DO in the strike water, mash, sparge, boil, chill and transfer of wort into the fermenter. So why, then, do we dump a crap-ton load of O2 into the
wort when we pitch the yeast? It's the
yeast that needs the O2. So doesn't it make more sense to saturate the
starter with O2, and wait till high krausen when nearly all of that O2 has been metabolized and the highly active yeast colony is starving for the abundant sugars in the fresh wort?
The yeast don't need oxygen to either ferment the wort or propagate their colony. They do need O2 to kickstart their metabolism out of its dormant state however, which is what we do when we create a starter. I get it that any O2 in the wort will be consumed within 30 minutes to an hour of pitching, but there are those here who are obsessing over a bottle-capful of air getting into a fermenter for a few seconds and 'destroying' the beer. I'm not disputing that fact, but rather trying to resolve the apparent ambiguity.
If DO is 'bad' and eliminating it is 'good' (with which I totally agree), but O2 is necessary in the initial propagation of yeast but not required in the fermentation of the wort, then why do we oxygenate the wort? I'm not advocating a radical departure from conventional wisdom or questioning new LoDO methodologies. I know there must be a simple and obvious reason that I'm overlooking. If so, please point it out in a legitimate and reasonable way, in the same manner in which the question is asked.
Brooo Brother