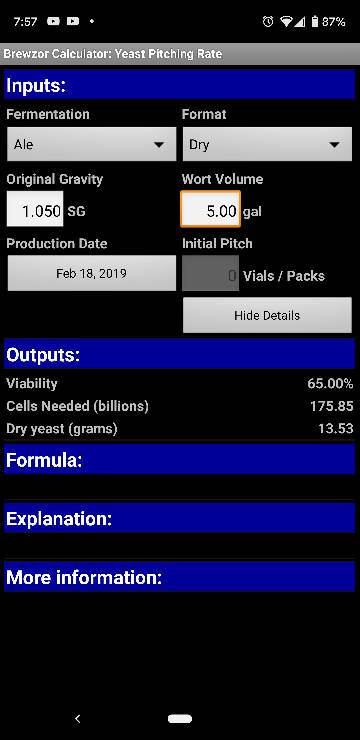
You're not entirely correct there. Yeast do need O2 in order to propagate as oxygen is required for sterol synthesis which is a fundamental component of cell mebranes. Yeast does not need O2 to kickstart fermentation. What kickstarts fermentation are the simple sugars (meaning glucose, fructose and saccharose) that are present in malt as a leftover of the malting process.
In conventional fermentations yeast will propagate by a factor of 4 up to 5 times the initial pitch rate. Oxygen is required for that as yeast will need sterols to bud repeatedly while producing healthy daughter cells.
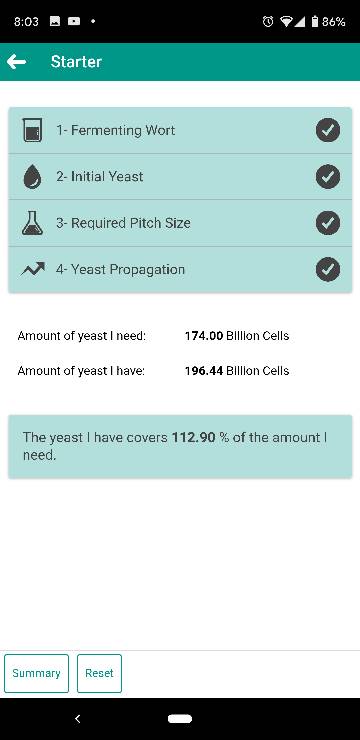
It is possible and there is research being done into oxygen-free pitching but the only practical way to achieve this seems to be to pitch so much healthy, vital yeast that propagation after pitching is no longer necessary for a rapid, trouble-free fermentation. Pressure fermentation will also be needed to fully suppress yeast propagation after pitching. This will however impact the fermentation profile in very complicated ways so in reality this is not as trivial as it might sound.
In conventional fermentations yeast will propagate by a factor of 4 up to 5 times the initial pitch rate. Oxygen is required for that as yeast will need sterols to bud repeatedly while producing healthy daughter cells.
It is possible and there is research being done into oxygen-free pitching but the only practical way to achieve this seems to be to pitch so much healthy, vital yeast that propagation after pitching is no longer necessary for a rapid, trouble-free fermentation. Pressure fermentation will also be needed to fully suppress yeast propagation after pitching. This will however impact the fermentation profile in very complicated ways so in reality this is not as trivial as it might sound.