You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Water adjustment sanity-check
- Thread starter jackbflyin
- Start date

Help Support Homebrew Talk - Beer, Wine, Mead, & Cider Brewing Discussion Forum:
This site may earn a commission from merchant affiliate
links, including eBay, Amazon, and others.
ApolloSimcoe
Well-Known Member
I'm no water expert but if it were me i would ditch the Baking Soda, i mean all of it. I believe Its counter productive to the gypsum and calcium chloride. If you need to lower your mash pH try lactic acid or acidulated malt.
Baking Soda is used more commonly in darker beers
I'm sure someone will jump in with a more robust answer for you.
Baking Soda is used more commonly in darker beers
I'm sure someone will jump in with a more robust answer for you.
jackbflyin
Active Member
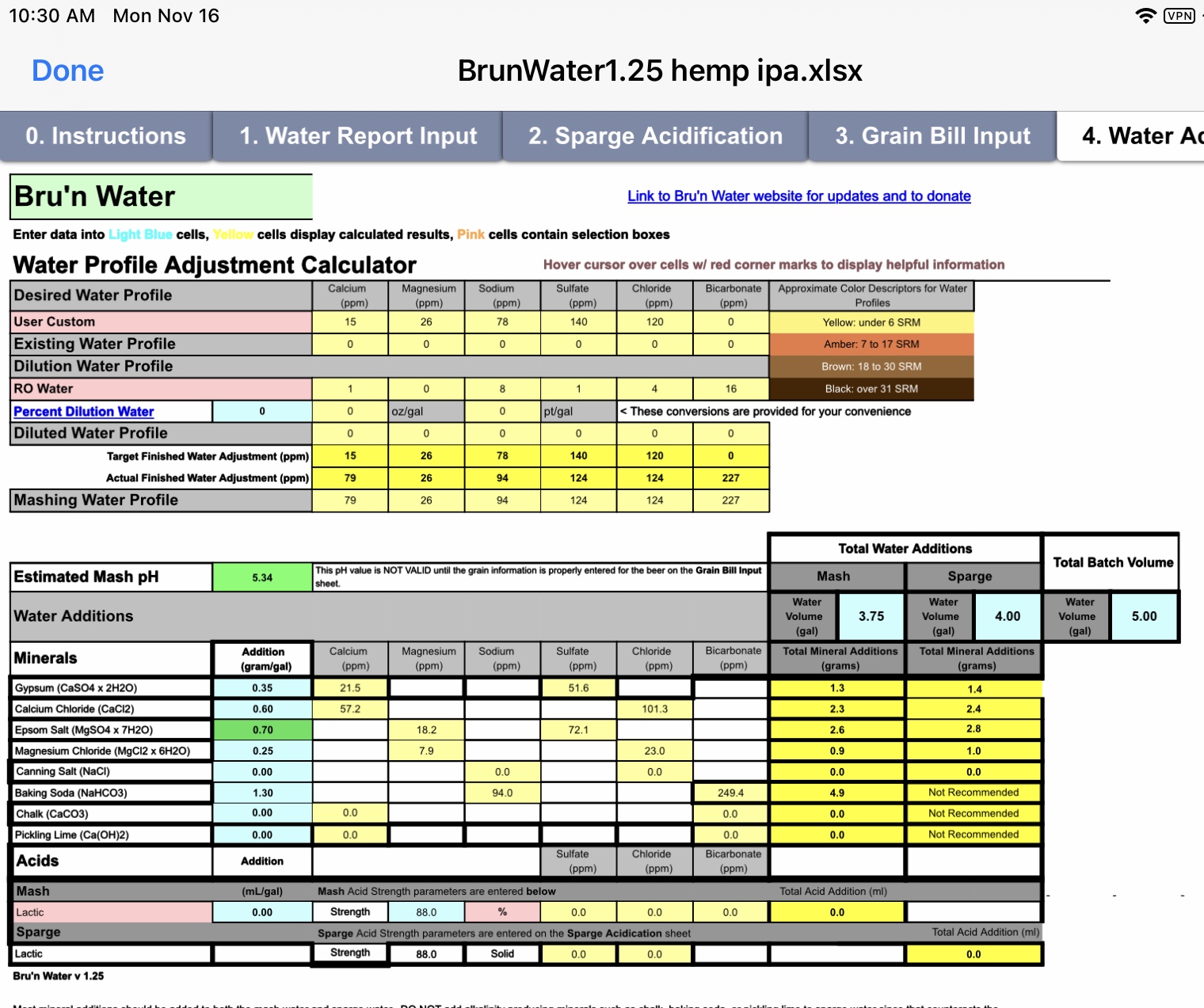
my pH is too low so I added the baking soda to bring it up to 5.3. Should I use something different?
my pH is too low so I added the baking soda to bring it up to 5.3. Should I use something different?
I'm curious to learn what grain bill would produce a mash pH < 5.3 with that water profile.
Gnomebrewer
Well-Known Member
I'm no water expert but if it were me i would ditch the Baking Soda, i mean all of it. I believe Its counter productive to the gypsum and calcium chloride. If you need to lower your mash pH try lactic acid or acidulated malt.
Baking Soda is used more commonly in darker beers
I'm sure someone will jump in with a more robust answer for you.
In a darker beer, the baking soda would still be used alongside the gypsum and Calcium chloride. They aren't counter productive (gypsum and CaCl2 are in there for flavour but also happen to lower pH slightly). Using baking soda alongside an acid is counterproductive. In this case there is no reason to use it - a pale beer will need acid (not alkalinity) to get to a suitable pH.
my pH is too low so I added the baking soda to bring it up to 5.3. Should I use something different?
No. There must be something wrong with your input if your pH estimate is too low. You'll need acid, not alkalinity. Without acid the pH will be too high.
Gnomebrewer
Well-Known Member
Can you post a shot of your grain input tab?
It's possible that the mashing water needs some alkalinity if the calcium content is high and the grist is a bit amber. 79 ppm Ca is a bit high and adding that much Mg is higher than I would go. The other thing that concerns me is the amount of sulfate in what I'm assuming is a NEIPA. The chloride level is fine, but the sulfate should be more on the order of 75 ppm.
Adding baking soda is unexpected in pale beers, but it's possible to be needed. One caution is that, dry hopping beer with a lot of hops tends to increase beer pH. But that doesn't mean that you should allow the wort pH to be less than about 5.3 since that can reduce the body of the beer. Target a proper pH in the 5.3 to 5.5 range. Don't be surprised if the beer needs some post-fermentation acid in order to improve the flavor and perception.
Adding baking soda is unexpected in pale beers, but it's possible to be needed. One caution is that, dry hopping beer with a lot of hops tends to increase beer pH. But that doesn't mean that you should allow the wort pH to be less than about 5.3 since that can reduce the body of the beer. Target a proper pH in the 5.3 to 5.5 range. Don't be surprised if the beer needs some post-fermentation acid in order to improve the flavor and perception.
Gnomebrewer
Well-Known Member
It's possible that the mashing water needs some alkalinity if the calcium content is high and the grist is a bit amber.
Adding baking soda is unexpected in pale beers, but it's possible to be needed.
The beer is a treehouse julius clone. I've never seen pale crystal in one, let alone something dark enough to cause a large pH drop (and no roasted malts). It's only 79ppm Calcium (I'd call that low to moderate for an ale) and is calling for 248ppm bicarbonate in the mash! That's not right. I'd still like to see the grist input - I think we'll see an error there (with the data input, not the spreadsheet).
Similar threads
- Replies
- 52
- Views
- 2K
- Replies
- 1
- Views
- 645
- Replies
- 6
- Views
- 1K