Knocking off a quick batch of Guinness per my son's request with the 70:20:10 (MO:flaked barley:roast barley 550L) mash. I've seen two main houses of opinions as to water used; I'd prefer to go with the moderately hard water that was from the Grand Canal, per commentary by @cire.
Sorry, lazy request. Just looking for this older Guinness mineral content, and some suggestions as to mash and sparge water given my water. I have lots to catch up on (I mean, brewing science, even rudimentary stuff as I've forgotten everything) and wanted to get this beer going for him on a winter storm tomorrow.
Please note I won't be doing any sour-blending or the like. Just a dry stout with some qualities shared with draft Guinness.
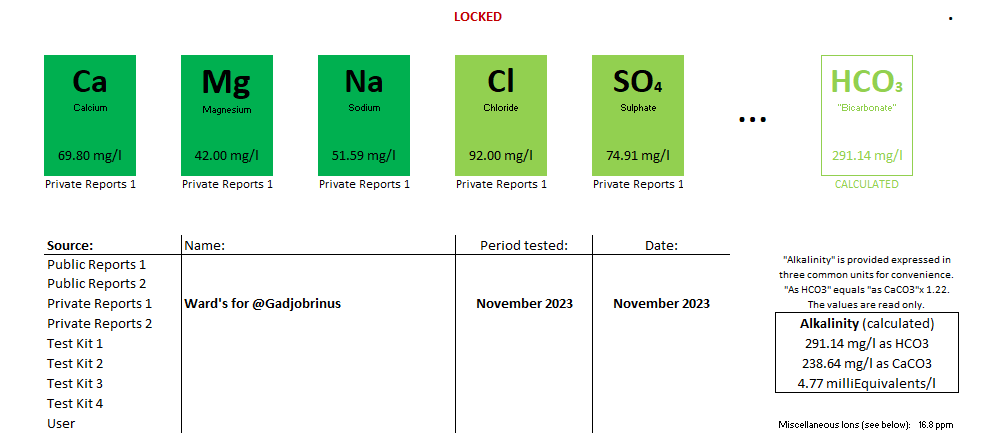
Here's my water.
Ca: 69.8
Mg: 42.0
Na: 49.0
HCO3: 291.0
SO4: 25.0
Cl: 92.0
Alkalinity as CaCO3: 239
For the mash, I was thinking of doing nothing but adding in some gypsum to get SO4 and Cl at rough equity.
For the sparge, I was thinking of doing 50:50 my water and RO, then just using lactic 88% to get the pH down to 5.7. Any lactic "bite" would just be a bit of a cheat on the Guinness tang (don't think there would be any lactic bite, after the dilution, given the amount needed).
Thoughts?
Sorry, lazy request. Just looking for this older Guinness mineral content, and some suggestions as to mash and sparge water given my water. I have lots to catch up on (I mean, brewing science, even rudimentary stuff as I've forgotten everything) and wanted to get this beer going for him on a winter storm tomorrow.
Please note I won't be doing any sour-blending or the like. Just a dry stout with some qualities shared with draft Guinness.
Here's my water.
Ca: 69.8
Mg: 42.0
Na: 49.0
HCO3: 291.0
SO4: 25.0
Cl: 92.0
Alkalinity as CaCO3: 239
For the mash, I was thinking of doing nothing but adding in some gypsum to get SO4 and Cl at rough equity.
For the sparge, I was thinking of doing 50:50 my water and RO, then just using lactic 88% to get the pH down to 5.7. Any lactic "bite" would just be a bit of a cheat on the Guinness tang (don't think there would be any lactic bite, after the dilution, given the amount needed).
Thoughts?