Hello All,
I've posted some questions into an other thread and i was routed to here.
I'm using Bru'n water calculator. But something is wrong or i do not know.
So i'm facing some problems (based on info i've received at the other thread)
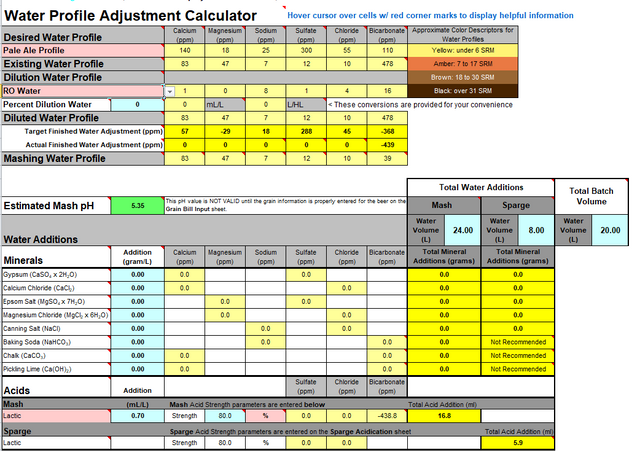
-my tapwater is too hard (too high bicarbonate and CaO levels)
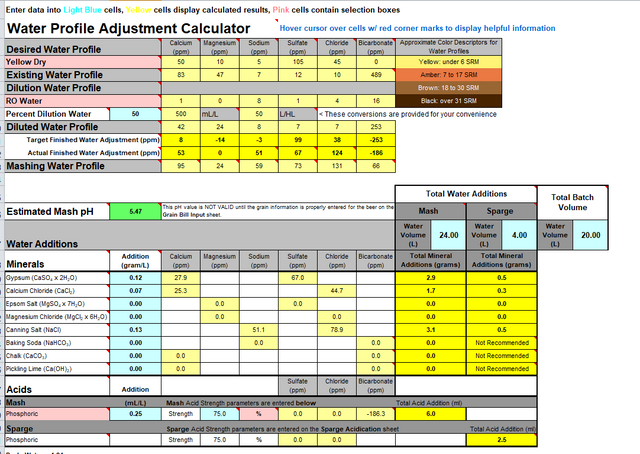
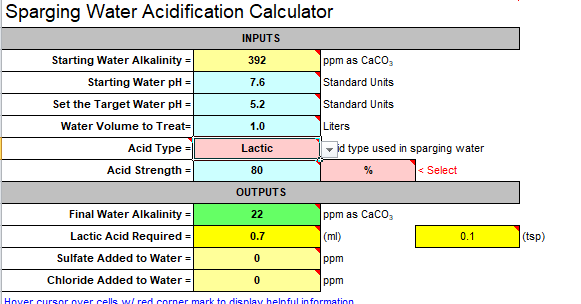
-for some reason the calculator said i need 0.7-0.8ml of 80% lactic acid / liter to lower the ph which amount was questioned at the other thread (0.7ml/L is too high volume)
May I ask some help to target a profile with this calculator which is suitable for NEIPA brewing via biab method?
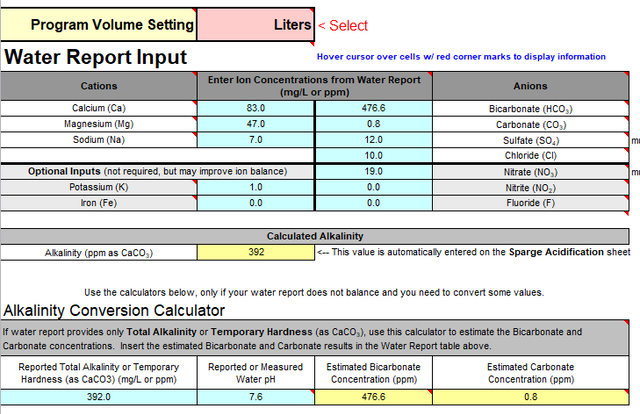
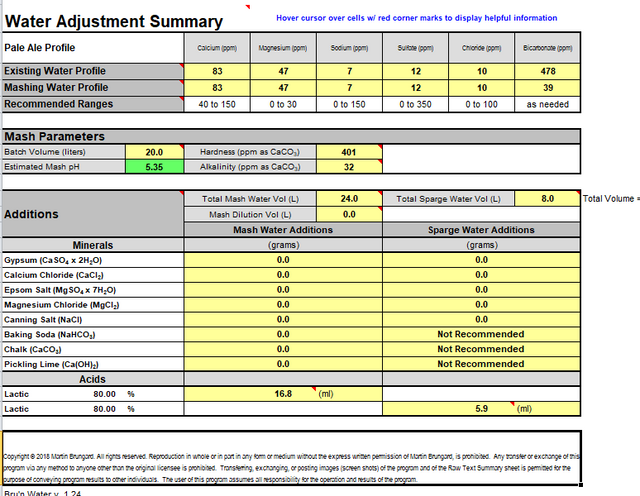
My tapwater profile:
-Ca: 83 mg/L
-K: 1 mg/L
-Cl: 10 mg/L
-Mg: 47.1 mg/L
-Na: 7 mg/L
-Nitrate: 19mg/L
-Nitrite: <0.01mg/L
-Fe: 0.04 mg/L
-Sulfate: 12mg/L
-Nk: 22
-Ph: 7.56
Here are some pictures of the calculator and some info about value "NK" (it was told to me to bring this info to this question too)
"
By the way, I found a source for the meaning of the "Nk" value, for those who were wondering:
Water hardness
The pleasure value of potable water and efficiency of the water used for washing are determined in part by the hardness of water, that is, its CaO (calcium oxide) mg/litre ratio. Water hardness figures indicate that in the region of the capital the water is mostly of medium hardness. It is important to know that the harder the water, the more pleasant flavourit has, but the lesser it is suitable for washing, and our washing machine does not like hard water. In most of the cases – e.g. on the labels of the detergents – water hardness is indicated in terms of the German standard of hardness (nk)*.
*The German standard for hardness (nk) is one tenth of the CaO mg/litre, so for 141 CaO mg/litre, for example, this value is 14.1 nk (2016).
Pass this information on to the Brew Science forum when posting your question there, together with screenshots of the new Bru'n Water calculations.
The originators/builders of Bru'n Water are on the Brew Science forum, they can help you with any questions on the matter.
Remember, I mentioned the missing Anions from your water report? Well we found those, and they are indeed in the alkalinity. You have fairly hard waterwhich may not be all that suitable for brewing, as is, so some tweaking is definitely needed.
"

 [/url]
[/url]
I've posted some questions into an other thread and i was routed to here.
I'm using Bru'n water calculator. But something is wrong or i do not know.
So i'm facing some problems (based on info i've received at the other thread)
-my tapwater is too hard (too high bicarbonate and CaO levels)
-for some reason the calculator said i need 0.7-0.8ml of 80% lactic acid / liter to lower the ph which amount was questioned at the other thread (0.7ml/L is too high volume)
May I ask some help to target a profile with this calculator which is suitable for NEIPA brewing via biab method?
My tapwater profile:
-Ca: 83 mg/L
-K: 1 mg/L
-Cl: 10 mg/L
-Mg: 47.1 mg/L
-Na: 7 mg/L
-Nitrate: 19mg/L
-Nitrite: <0.01mg/L
-Fe: 0.04 mg/L
-Sulfate: 12mg/L
-Nk: 22
-Ph: 7.56
Here are some pictures of the calculator and some info about value "NK" (it was told to me to bring this info to this question too)
"
By the way, I found a source for the meaning of the "Nk" value, for those who were wondering:
Water hardness
The pleasure value of potable water and efficiency of the water used for washing are determined in part by the hardness of water, that is, its CaO (calcium oxide) mg/litre ratio. Water hardness figures indicate that in the region of the capital the water is mostly of medium hardness. It is important to know that the harder the water, the more pleasant flavourit has, but the lesser it is suitable for washing, and our washing machine does not like hard water. In most of the cases – e.g. on the labels of the detergents – water hardness is indicated in terms of the German standard of hardness (nk)*.
- Very soft water: under 40 CaO mg/litre (4 nk)
- Soft water: between 40-80 CaO mg/litre (4-8 nk)
- Medium-hard water: between 80-180 CaO mg/litre (8-18 nk)
- Hard water: between 180-300 CaO mg/litre (18-30 nk)
- Very hard water: above 300 CaO mg/litre (30 nk)
*The German standard for hardness (nk) is one tenth of the CaO mg/litre, so for 141 CaO mg/litre, for example, this value is 14.1 nk (2016).
Pass this information on to the Brew Science forum when posting your question there, together with screenshots of the new Bru'n Water calculations.
The originators/builders of Bru'n Water are on the Brew Science forum, they can help you with any questions on the matter.
Remember, I mentioned the missing Anions from your water report? Well we found those, and they are indeed in the alkalinity. You have fairly hard waterwhich may not be all that suitable for brewing, as is, so some tweaking is definitely needed.
"