I don't believe that that method would provide you with satisfactory results. You still need to be able to assess and accommodate your water's variation.
You aren't grasping the essence of the 0 Alkainity method. I obviates the need to compensate for water's variation with respect to alkalinity. You do not need to assess the water's alkalinity. That is done implicitly within the method as is the "calculation" of the acid amount needed to neutralize to mash pH. The acid needed for the water and the acid needed for the malt are entirely separable. The 0 Alkalinity method takes care of the water part with one exception that I will get to in a minute. I strongly suspect that one of the problems with first generation spreadsheets is that they try to couple malt and water alkalinity perhaps through residual alkalinity but I really have no idea how they work. I also suspect that this is responsible for some of the spectacularly wild answers they give.
A second generation spreadsheet understands that there is a proton deficit associated with the water, a proton deficit assignable to the alkalinity in it, a proton deficit for each malt, computes them separately, sums them up and either adjusts an acid addition or malt amount to set this sum to 0 when pH control is the goal or varies pH to get 0 deficit when pH is being estimated. The 0 alkalinity method works by setting the deficit of the water and the bicarboate/carbonate in it to 0 by the simple expedient of setting the water's pH to the target mash pH.
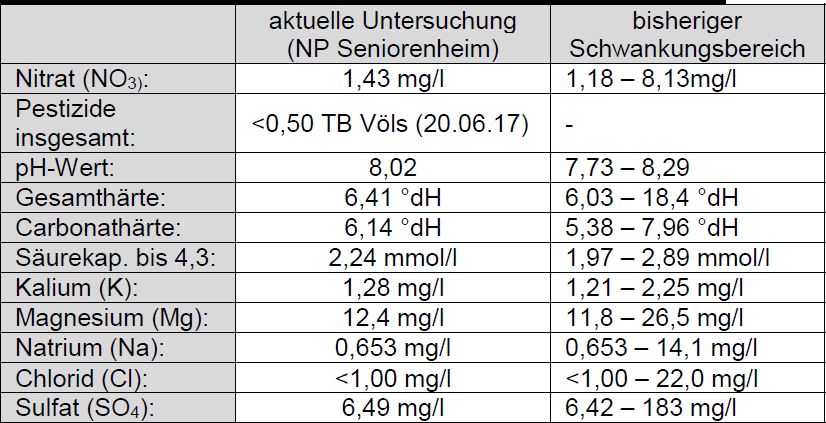
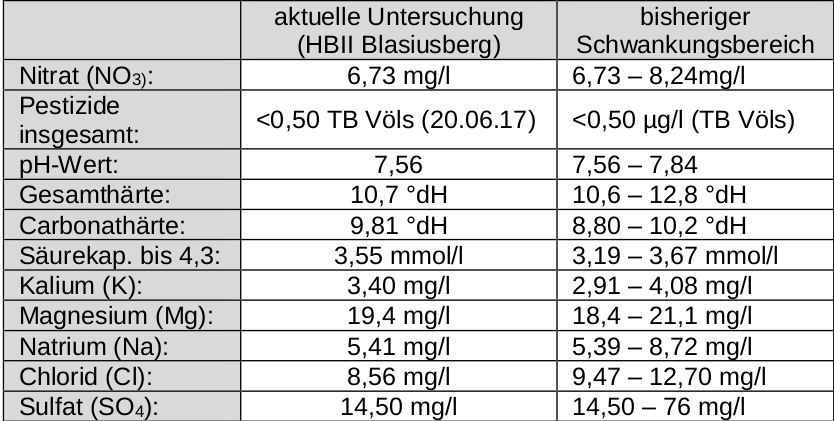
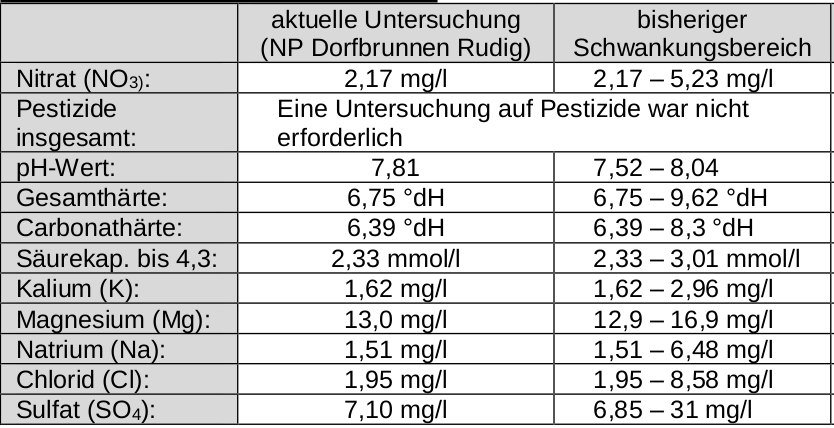
What I said I'd come back to: the OP's water varies somewhat widely in alkalinity. The 0 Alkalinity method takes care of that. It does not take care of the huge variation in sulfate. Sulfate is difficult for a home brewer to test for except grossly (precision of 50 mg/L) so either one must accept that he will not know where his sulfate falls or swamp it with huge additions which will not be suitable for many styles. It will also not compensate for the small variations in mash pH attributable to calcium variation. Still more on that later.
If you plan on using your water supply, I recommend that you contact the water supplier and ask for the water parameters from the two water sources (hopefully its only two) and then you can enter them into a calculator such as Bru'n Water to better estimate what your current water quality is.
Unless you know which source is being drawn from or in what proportion the two (or more) sources are being blended, a spreasheet, even a second generation one, will do you no good.
Unfortunately, you'll need the supporter's version to do this blending exercise easily.
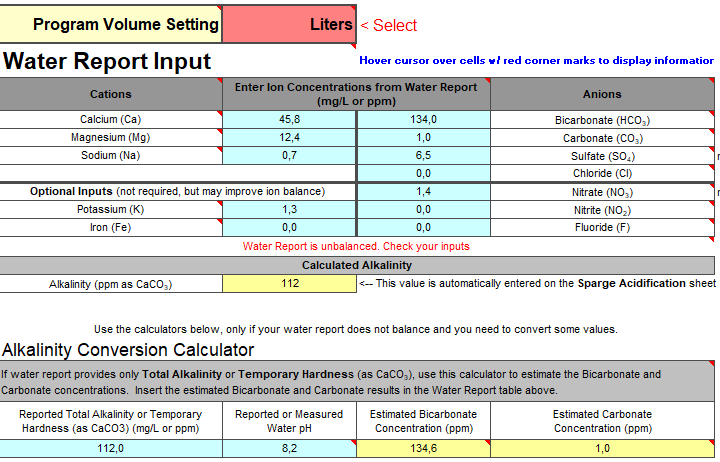
Fortunately there are free calculators coming on line that will do a better job of calculating the properties of blended waters. Most of the first generation ones don't even ask about the waters' pH values or process reported alkalinity correctly. This is mooted if the 0 alkalinity method is used.
You should be able to employ two relatively simple and inexpensive testing kits for calcium hardness and alkalinity and use those results to vary the percentages of the two (I'm hoping) water sources within the water calculator until the blended water better matches your current water measurements.
But how does he know how much of what the utility is blending and how does he get the separate source samples to test? More practical for him to test the water that comes out of the tap on brew day (perhaps that's what you meant to say). Or use the 0 alkalinity method which doesn't care.
Now I firmly endorse the idea of obtaining the tests and checking the water each time one brews. The more you know about your water the better. Even if using the 0 Alkalinity method you will want to know the calcium and magnesium hardnesses in order that you can estimate the protons those ions produce in the mash. Those protons get deducted from the total mash proton deficit - 0 alkalinity method or otherwise.
That way, you have a better estimate of your starting water conditions and will be able to get closer to your water and mashing targets.[/QUOTE]