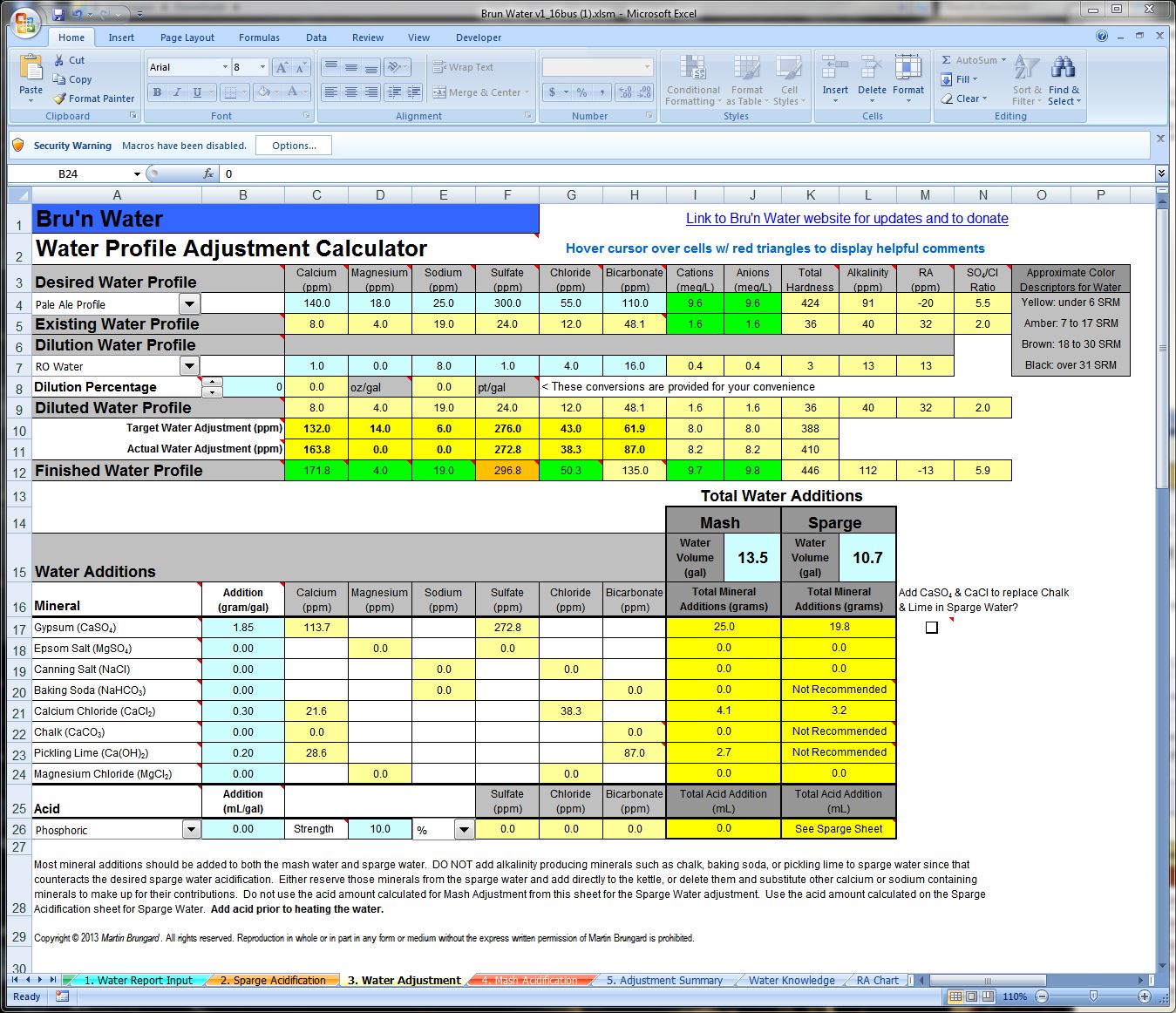
Got my ward's lab report today, and am trying to determine adjustments for a 15 gallon batch clone of Sierra Nevada Celebration Ale. My HLT holds 24 gallons, so I've used Beersmith2 to calculate the additions for this volume of water.
My tap water (from Chesterfield County, Virginia) has:
pH 7.4
Total Dissolved Solids (TDS) Est, ppm 110
Electrical Conductivity, mmho/cm 0.18
Cations / Anions, me/L 1.6 / 1.7
ppm
Sodium, Na 19
Potassium, K 2
Calcium, Ca 8
Magnesium, Mg 4
Total Hardness, CaCO3 37
Nitrate, NO3-N < 0.1 (SAFE)
Sulfate, SO4-S 8
Chloride, Cl 12
Carbonate, CO3 < 1
Bicarbonate, HCO3 48
Total Alkalinity, CaCO3 39
Total Phosphorus, P 0.38
Total Iron, Fe < 0.01
If I want to match the water profile of Vista, CA (which some thread research on hbt seems to suggest may be slightly less bitter than Mosher's), Beersmith2 says I should add the following to my HLT (which again holds 24 gallons):
Gypsum 9.3g
Table Salt 6.5g
Epsom Salt 16.6g
Calcium Chloride 5.8g
Baking Soda 7.9g
Chalk 1.7g
The additions will result in a profile of:
Ca 57ppm
Mg 22ppm
Na 71ppm
SO4 136ppm
Cl 88ppm
HCO3 122ppm
I have never played around with water chemistry before, as I usually brew dark beers and have been happy with their taste. My IPAs are not as "crisp" as I'd like for them to be, so I started reading up a little and got a water test.
Appreciate any thoughts/suggestions.
My tap water (from Chesterfield County, Virginia) has:
pH 7.4
Total Dissolved Solids (TDS) Est, ppm 110
Electrical Conductivity, mmho/cm 0.18
Cations / Anions, me/L 1.6 / 1.7
ppm
Sodium, Na 19
Potassium, K 2
Calcium, Ca 8
Magnesium, Mg 4
Total Hardness, CaCO3 37
Nitrate, NO3-N < 0.1 (SAFE)
Sulfate, SO4-S 8
Chloride, Cl 12
Carbonate, CO3 < 1
Bicarbonate, HCO3 48
Total Alkalinity, CaCO3 39
Total Phosphorus, P 0.38
Total Iron, Fe < 0.01
If I want to match the water profile of Vista, CA (which some thread research on hbt seems to suggest may be slightly less bitter than Mosher's), Beersmith2 says I should add the following to my HLT (which again holds 24 gallons):
Gypsum 9.3g
Table Salt 6.5g
Epsom Salt 16.6g
Calcium Chloride 5.8g
Baking Soda 7.9g
Chalk 1.7g
The additions will result in a profile of:
Ca 57ppm
Mg 22ppm
Na 71ppm
SO4 136ppm
Cl 88ppm
HCO3 122ppm
I have never played around with water chemistry before, as I usually brew dark beers and have been happy with their taste. My IPAs are not as "crisp" as I'd like for them to be, so I started reading up a little and got a water test.
Appreciate any thoughts/suggestions.