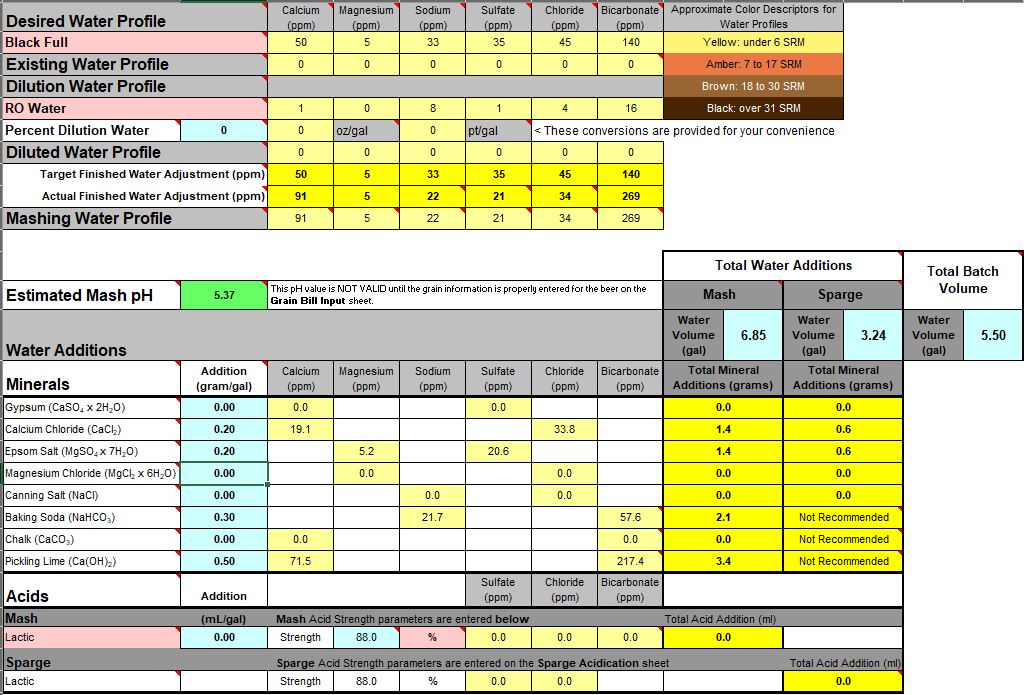
Think of it this way. If for baking soda only 427 ppm of bicarbonate is required to bring the pH up to 5.5, then why should it take 600 ppm of slaked lime induced bicarbonate (a misnomer, since there is no carbonate species present within slaked lime, but lets bypass that for now) to accomplish the same thing? As I've been saying, something is wrong. (I also disagree with 427 ppm bicarbonate for 15.2 grams of baking soda, as I see it as 497 ppm, but lets also bypass that issue for now)
I'm trying to get you to think this through logically as well as mathematically....
Now lets focus on that predicted 4.51 pH before adding any baking soda and/or slaked lime addition. There are only a small handful of caramel/crystal and deep roasted malts which have stand alone mash pH's in DI water that go below 4.51 pH. And base malts are closer overall to about 5.7 DI_pH, and flaked oats are in the pH 6's. So logically it is only possible to mash at 4.51 pH if your entire recipe (or grist) consisted of something like 100% black barley (DI_pH ~4.59), plus some downward pH shift due to added calcium and/or magnesium mineralization. Your grist is not 100% black barley.
Again, the exercise I'm presenting here is to think this through both logically and mathematically.... And again, something is wrong. Think outside of the box. What is the source of these problems?