Quick question which has probably already been answered - with the fermentation gas purging, does the keg need to be tilted with the gas post the highest point to get all of the O2 out? (like a liquid purge). I have been purging for almost a year but noticed I am just standing the keg straight upright which allows the lid to trap some things. Thanks.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Keg purging with active fermentation
- Thread starter Mer-man
- Start date

Help Support Homebrew Talk - Beer, Wine, Mead, & Cider Brewing Discussion Forum:
This site may earn a commission from merchant affiliate
links, including eBay, Amazon, and others.
No, you don't need to tilt the keg being purged. The analysis doesn't depend on any particular flow pattern being required.Quick question which has probably already been answered - with the fermentation gas purging, does the keg need to be tilted with the gas post the highest point to get all of the O2 out? (like a liquid purge). I have been purging for almost a year but noticed I am just standing the keg straight upright which allows the lid to trap some things. Thanks.
Brew on

Thanks for your quick reply. Gas exchange is pretty mystical to me. I would have thought having some sort of funnel situation would make it easier to direct flow outward and avoid eddys like in water. At a base level, would tilting improve the efficiency?
Again no. If the process were marginal, then trying to optimize flow patterns might make a difference, but this process is so effective, that there isn't really anything to do to improve it significantly. Don't complicate things that won't benefit from it.Thanks for your quick reply. Gas exchange is pretty mystical to me. I would have thought having some sort of funnel situation would make it easier to direct flow outward and avoid eddys like in water. At a base level, would tilting improve the efficiency?
Brew on

Thanks!
kurds_2408
Well-Known Member
- Joined
- Dec 19, 2013
- Messages
- 207
- Reaction score
- 153
Don’t know if anyone saw my last post as it was the last post before a new page just a couple hours after. But planning to copy the carb cap on the bucket lid trick that was posted earlier. This is my first time researching closed transfers so had a question.
Currently all my ferm buckets have bottling spigots on them and and I transfer to kegs with gravity instead of using an autosiphon. Since I have spigots already instead of doing two carb caps on the lid with one having a dip tube, could I only do one carb cap on the lid for purging the keg during ferm, then run a tube from the spigot to the beer in post on the keg, then hook up CO2 to the carb cap in the lid and apply like 1psi to it during a gravity transfer? Not necessarily to pressure transfer but more to fill the head space as it gravity transfers to the keg. This would be a completely closed system still I think.
Currently all my ferm buckets have bottling spigots on them and and I transfer to kegs with gravity instead of using an autosiphon. Since I have spigots already instead of doing two carb caps on the lid with one having a dip tube, could I only do one carb cap on the lid for purging the keg during ferm, then run a tube from the spigot to the beer in post on the keg, then hook up CO2 to the carb cap in the lid and apply like 1psi to it during a gravity transfer? Not necessarily to pressure transfer but more to fill the head space as it gravity transfers to the keg. This would be a completely closed system still I think.
Last edited:
What I have been doing is a line from my spigot to the liquid post on the keg, and a line from the gas post on the keg to the top of my fermenter. I then use gravity for the transfer. The CO2 from the purged keg replaces the headspace in the fermenter. What you describe should work just fine as well, with the downside that you use a bit of CO2 to fill the fermenter.Currently all my ferm buckets have bottling spigots on them and and I transfer to kegs with gravity instead of using an autosiphon. Since I have spigots already instead of doing two carb caps on the lid with one having a dip tube, could I only do one carb cap on the lid for purging the keg during ferm, then run a tube from the spigot to the beer in post on the keg, then hook up CO2 to the carb cap in the lid and apply like 1psi to it during a gravity transfer? Not necessarily to pressure transfer but more to fill the head space as it gravity transfers to the keg. This would be a completely closed system still I think.
As far as the design of your caps, how do you plan to attach them to the bucket lid? Are they designed to take a carbonation cap?
kurds_2408
Well-Known Member
- Joined
- Dec 19, 2013
- Messages
- 207
- Reaction score
- 153
Man that’s brilliant. I’m definitely doing that. Such a better use of otherwise wasted CO2. Thanks. And yeah that design I made is just soda bottle threads. So use it with the carb caps to sandwhich the lid and rubber washers. Only bad thing is i run pin locks so gotta get some ball connectors to mix and match.What I have been doing is a line from my spigot to the liquid post on the keg, and a line from the gas post on the keg to the top of my fermenter. I then use gravity for the transfer. The CO2 from the purged keg replaces the headspace in the fermenter. What you describe should work just fine as well, with the downside that you use a bit of CO2 to fill the fermenter.
As far as the design of your caps, how do you plan to attach them to the bucket lid? Are they designed to take a carbonation cap?
kurds_2408
Well-Known Member
- Joined
- Dec 19, 2013
- Messages
- 207
- Reaction score
- 153
Got my Carb Caps in and my threaded adapter printed. My hardware store didn’t have the correct size rubber washer but did have rubber washer making sheets. Well in addition to 3D printing I play with lasers so made me some rubber washers with the laser cutter. Worked perfectly. Super excited to ferm purge my next batch.









DuncB
Well-Known Member
Perhaps add it into this thread.Got my Carb Caps in and my threaded adapter printed. My hardware store didn’t have the correct size rubber washer but did have rubber washer making sheets. Well in addition to 3D printing I play with lasers so made me some rubber washers with the laser cutter. Worked perfectly. Super excited to ferm purge my next batch.
View attachment 823998View attachment 823999View attachment 824000View attachment 824001View attachment 824002
https://www.homebrewtalk.com/threads/homebrew-talk-3d-print-thread.596124/page-6#post-10272580
kurds_2408
Well-Known Member
- Joined
- Dec 19, 2013
- Messages
- 207
- Reaction score
- 153
Oh good point. I’ll do that now.Perhaps add it into this thread.
https://www.homebrewtalk.com/threads/homebrew-talk-3d-print-thread.596124/page-6#post-10272580
A ball lock keg has a total volume of about 5.3 gal or 678 fl oz. Due to the lid design, there is a volume of 3 fl oz that cannot be filled with liquid (for an unmodified keg.) When you then blow the liquid out of the keg with CO2, that 3 fl oz of air is dispersed throughout the entire keg volume. Thus the "starting" O2 concentration is:
210,000 ppm * 3 / 678 = 930 ppmSo, doing a liquid purge is almost equivalent to doing 5 purge cycles at 30 psi. Thus you still need 8 more 30 psi purge cycles to get down to the ~100 ppb O2 range.
Brew on
if you fill the corny to the rim then there's no way for the full 3fl oz of air to get into the keg. due to the shape of the lid opening, you easily submerge at least half of the lid, probably closer to 2/3 of it if you're careful and deliberate. so its more like 1-ish fl oz of air trapped in there? the water (at the rim level) basically hits the lid right around where the prv stem is, which is roughly 2/3 of the lid area i'd wager.
so assuming that we're down to more like 310ppm when you start your liquid purge. by the math quoted above.
although looking at the purge chart, (didnt see the math to calculate specifically) it still seems like you'd need 8 purges at 30 to get to 50ppb....
just realized that since the lid sits above the rim of the keg, even if the lid is submerged as soon as you manuever the lid upwards, air must get sucked in to fill the void. so the premise i had initially is wrong. its the full 3oz of air.
Last edited:
been nerding out on this for a while, figuring out how bad i've been leaving o2 around because i've been putting a spunding valve on my serving keg. the 2psi makes sure the lid stays sealed tight, which was a problem once before. however i believe that has the practical effect of massively increasing the "volume of co2" i need to do the purge. numbers are really bad.....Ok, a "5" gal corny has an actual volume of about 5.35 gal, so 2 of those plus 1.5 gal headspace in the fermenter totals 12.2 gal, or 46.2 liters. Assuming you have 20 liters of wort at 1.050 that finishes at 1.010, you would generate ~440 liters of CO2 during fermentation. After some multiple gas dilution cycles the final O2 concentration is about 15 ppb (0.015 ppm.) So, purging (2X) 5 gal kegs with a 5 gal ferment appears to give acceptable results.
Brew on
but in the process of checking my numbers, i re-ran the numbers you have above for the daisy-chain of two kegs. its essentially like what i'm doing above - but adding more volume instead of pressure.
i went back and re-checked everything in the spreadsheet (thanks to @Adam Zerwick ) with your original figures for 20L wort, 1.050 down to 1.010, with 20L headspace and everything matches. so i think the spreadsheet is good.
running the numbers you cite above with the extra volume i'm getting like 15727 ppb of oxygen. which is horrible.
is that right, or did we goof somewhere?
Last edited:
Not sure if you would actually end up with less air or not this way. The original statement assumed you filled a sealed keg thru the liquid post, with an open PRV valve, and an unconnected gas QD on the gas post. Might be possible to measure if there is a difference filling with the lid off and then closing up the keg, but you need a pretty accurate scale, about 0.1 oz resolution with a 52-53 lb total weight.was filling a keg with near boiling water to sanitize after a brett beer today, being careful about sealing it up, and i thought of this thread. i think this might be a pretty heavy overestimation.
if you fill the corny to the rim (assuming you also punch your poppets to get any diptubes filled with water) then there's no way for the full 3fl oz of air to get into the keg. due to the shape of the lid opening, you easily submerge at least half of the lid, probably closer to 2/3 of it if you're careful and deliberate. so its more like 1-ish fl oz of air trapped in there? the water (at the rim level) basically hits the lid right around where the prv stem is, which is roughly 2/3 of the lid area i'd wager.
so assuming that we're down to more like 310ppm when you start your liquid purge. by the math quoted above.
although looking at the purge chart, (didnt see the math to calculate specifically) it still seems like you'd need 8 purges at 30 to get to 50ppb....
I'm not sure what difference this would make in actual practice, as if you purge with ferm CO2, you don't need to worry about such things. What are you getting at?
The math is pretty simple:
C = C0 * (14.7 / (P + 14.7))^n
C = concentration after "n" purge cycles
C0 = starting concentration
P = CO2 gauge pressure
n = number of purge cycles
Brew on

Need a complete and concise description of what you are actually doing. I'm not seeing it from what you have posted already.running the numbers you cite above with the extra volume i'm getting like 15727 ppb of oxygen. which is horrible.
is that right, or did we goof somewhere?
Brew on

so here's the spreadsheet derived from your calcs and with your original figures back at start of thread. it all matches up with your figures from that first huge post. seems correct to me. (ignore first two rows, no relation or effect here)
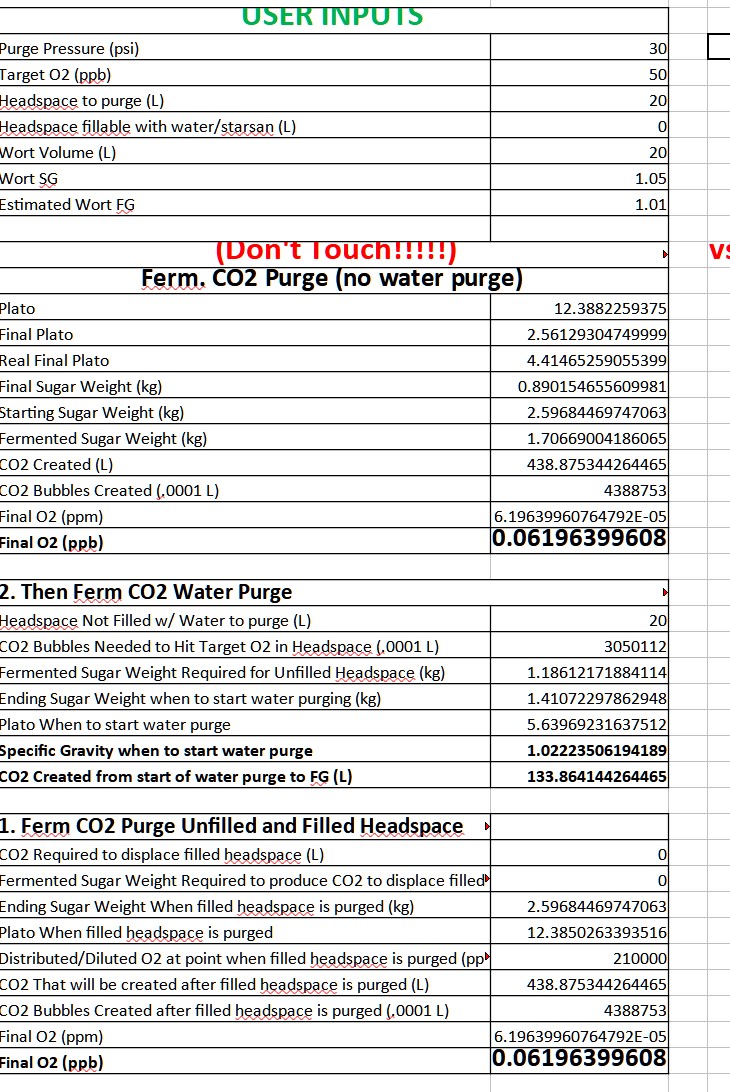
now here's changing the headspace volumes for two kegs and 1.5gal headspace, 46.2 liters per your post i cite above in #213. (again, ignore first two rows) same volume of co2 created, but now purging a little over twice the volume.


now here's changing the headspace volumes for two kegs and 1.5gal headspace, 46.2 liters per your post i cite above in #213. (again, ignore first two rows) same volume of co2 created, but now purging a little over twice the volume.

Last edited:
Red over White
Well-Known Member
Trimming the gas tubes and topping off the keg on an angle has made getting virtually all the air out of the keg easiest for me. Many times I will sanitize and then fill the keg (edit: to scrub 15 ppm oxygen) for a 5 gal keg I use 1.5g SMB) with Sodium Metabisulfite and let it sit for a couple hours before using the tail end of the ferment to push it out. This hack has worked well for me if I miss getting a keg hooked up at pitch.
Last edited:
so here's another question, and if the answer's here already by all means point it out- i didnt see it.
how do the purge charts account for co2 used to purge being impure ? here's an example. matheson, who supplies breweries and wineries locally, says they're equal or under 50ppm for food grade gas and 30ppm for beverage. thats 30000 to 50000ppb.
i assume it would need to be a fraction multiplied into the (14.7 + purge pressure) figure somehow......?
how do the purge charts account for co2 used to purge being impure ? here's an example. matheson, who supplies breweries and wineries locally, says they're equal or under 50ppm for food grade gas and 30ppm for beverage. thats 30000 to 50000ppb.
i assume it would need to be a fraction multiplied into the (14.7 + purge pressure) figure somehow......?
whattabrau
Well-Known Member
- Joined
- Apr 3, 2021
- Messages
- 116
- Reaction score
- 98
First, it's *minimum* purity, and O2 isn't the only possible contaminant.so here's another question, and if the answer's here already by all means point it out- i didnt see it.
how do the purge charts account for co2 used to purge being impure ? here's an example. matheson, who supplies breweries and wineries locally, says they're equal or under 50ppm for food grade gas and 30ppm for beverage. thats 30000 to 50000ppb.
i assume it would need to be a fraction multiplied into the (14.7 + purge pressure) figure somehow......?
Second, your pressure gauge reading inaccuracy completely dwarfs a >= 0.01% error by several orders of magnitude.
In other words, you *could* calculate that you need 5.0001 purge cycles instead of 5, but your pressure reading might be off and you actually need 4.5 or 5.5.
So, it doesn't affect the math to any meaningful degree.
This^First, it's *minimum* purity, and O2 isn't the only possible contaminant.
and that^Second, your pressure gauge reading inaccuracy completely dwarfs a >= 0.01% error by several orders of magnitude.
Also, if you're using commercial CO2 to carbonate and serve then the contaminating O2 in the CO2 tank is going to oxidize your beer even if you could somehow achieve a lower inital O2 concentration in the headspace.
whattabrau
Well-Known Member
- Joined
- Apr 3, 2021
- Messages
- 116
- Reaction score
- 98
True that. Given that the amount of CO2 in the headspace and beer is roughly equal per unit volume, the paranoid can calculate how much ~CO2 is drawn from the tank to carbonate ~50x the amount of the headspace.Also, if you're using commercial CO2 to carbonate and serve then the contaminating O2 in the CO2 tank is going to oxidize your beer even if you could somehow achieve a lower inital O2 concentration in the headspace.
And while we're riffing with the paranoid jam band, how much plain atmosphere do you have trapped between the gas disconnect and the post when you attach gas? It doesn't violently hiss when you push it on, so clearly it seals before the gas starts flowing, and whatever is trapped there goes into the keg.
I would love to see your math. You take 50 ppm o2 and shove it into a keg dozens of times and somehow it results in less than 50ppm? Very interesting. Please show your work.First, it's *minimum* purity, and O2 isn't the only possible contaminant.
Second, your pressure gauge reading inaccuracy completely dwarfs a >= 0.01% error by several orders of magnitude.
In other words, you *could* calculate that you need 5.0001 purge cycles instead of 5, but your pressure reading might be off and you actually need 4.5 or 5.5.
So, it doesn't affect the math to any meaningful degree.
Obviously not if you're purging an empty tank keg. But yes, if you're purging a tank keg that has 5 gallons of liquid and a quart of air, since whatever you ultimately leave in the headspace will equilibrate with the liquid. Final concentration ultimately also depends on how much O2 is dissolved in the liquid of course.
Yes, it's minimum purity (maximum possible impurities.) I saw a paper a while back (and stupidly didn't keep a copy or link) that analyzed actual O2 levels in beverage grade CO2. The typical O2 content was about 50 ppB, IIRC. I suspect that suppliers don't routinely measure the actual impurity content, as the equipment and procedures are much more expensive than what is required to demonstrate "no more than 30 ppM."First, it's *minimum* purity, and O2 isn't the only possible contaminant.
Second, your pressure gauge reading inaccuracy completely dwarfs a >= 0.01% error by several orders of magnitude.
In other words, you *could* calculate that you need 5.0001 purge cycles instead of 5, but your pressure reading might be off and you actually need 4.5 or 5.5.
So, it doesn't affect the math to any meaningful degree.
Brew on

Last edited:
Also, you need to convert from ppm or ppb by volume in the gas to ppm or ppb by weight in the liquid solution, since that's what is ultimately going to damage your beer (or not). Gas is much more diffuse than liquid, so 50 ppm in a minimal headspace after purging is not going to give anywhere near 50 ppm dissolved in your beer.
And while we're riffing with the paranoid jam band, how much plain atmosphere do you have trapped between the gas disconnect and the post when you attach gas?
Very little, I actually purge that. Tank on, ball valve on, press needle nose pliers into the disconnect for a few seconds. Burst that leftover air out (which is theoretically CO2 form the last time because I don't disconnect my lines, but it's PVC so I'm not counting on that for long).
I too fill the keg with a little theatrics, it's as full as I can get it without the lid, then the lid goes on, then it's tiled sideways to get the air out of the lid, and lastly it's finished being filled through the CO2 inlet.
I know it's not totally free form O2 but it's a little game I kind of like to play, and it's pretty close. As close as I'm reasonably going to get.
I do need to try the fermentation purge.
Nobody said anything about liquid. I’m talking about the gas purging charts. There’s no liquid involved.Also, you need to convert from ppm or ppb by volume in the gas to ppm or ppb by weight in the liquid solution, since that's what is ultimately going to damage your beer (or not). Gas is much more diffuse than liquid, so 50 ppm in a minimal headspace after purging is not going to give anywhere near 50 ppm dissolved in your beer.
The link I included for matheson is to their spec sheet for gas grades. It’s ppm. Post 218.Yes, it's minimum purity (maximum possible impurities.) I saw a paper a while back (and stupidly didn't keep a copy or link) that analyzed actual O2 levels in beverage grade CO2. The typical O2 content was about 50 ppB, IIRC. I suspect that suppliers don't routinely measure the actual impurity content, as the equipment and procedures are much more expensive than what is required to demonstrate "no more than 30 ppM."
Brew on :mug;
Also- look at matheson spec sheet. 50ppm for food grade. 30ppm for beverage. O2. Linked in post 218. The 0.1% of impurities is 1000ppm. Nobody said it was all o2.First, it's *minimum* purity, and O2 isn't the only possible contaminant.
Second, your pressure gauge reading inaccuracy completely dwarfs a >= 0.01% error by several orders of magnitude.
In other words, you *could* calculate that you need 5.0001 purge cycles instead of 5, but your pressure reading might be off and you actually need 4.5 or 5.5.
So, it doesn't affect the math to any meaningful degree.
Are you saying they are exaggerating their numbers? Because the idea that they would state 50ppm of o2 but give you gas with only 1ppm makes no sense. Why would they bother to even have those grades? The logical way to read it is that food grade is 30-50ppm. Beverage is X to 30ppm, where X is the max o2 level of the next higher grade.
OK, but why are you so worried about it? It's not an accurate represenntation of what is actually going to be impacting your beer.Nobody said anything about liquid. I’m talking about the gas purging charts. There’s no liquid involved.
As long as we're being this persnickety, the spec for their beverage grade is <= 30ppm; 50 ppm is the spec for the other grades.The link I included for matheson is to their spec sheet for gas grades. It’s ppm. Post 218.
Last edited:
No, the logical way to read it is that food grade is tested by a method with a limit of detection of 30 ppm and the other grades are tested by a method with a limit of detection of 50 ppm. At least that's what "<=" meant when I did product QA and QC for a living. If we had a test method that gave us a more precise result, we spec'd and reported the product that way.50ppm for food grade. 30ppm for beverage. O2. ...
... the idea that they would state 50ppm of o2 but give you gas with only 1ppm makes no sense. Why would they bother to even have those grades? The logical way to read it is that food grade is 30-50ppm. Beverage is X to 30ppm, where X is the max o2 level of the next higher grade.
Red over White
Well-Known Member
Ask yourself this. How many vessels are filled in the supply chain before your tank is actually filled? Are they purging the co2 pump to tank connection 100% before each fill. I 1000% doubt it and if you look at the open ended rig used to fill your tank, a shipload of air is going in first and then the co2. I've watched it firsthand in horror.
Well, ok, all of that apocryphal stuff could be true, but I purge kegs with fermentation gas (usually - otherwise it's Star San Purge city) and carbonate with CO2 from Airgas via my 20# siphon tank distributed to my collection of 5 pounders...and I routinely have the most O2-vulnerable beers stay bright and juicy for 6 months or more. Like the NEIPA I'm enjoying now - brewed in February.
As a design engineer for almost 50 years I was required to take worst-case specs into account, but as a home brewer with almost 20 years of experience I allow myself to accept the "usual case" and not wrap myself around the specification axle...
Cheers!
As a design engineer for almost 50 years I was required to take worst-case specs into account, but as a home brewer with almost 20 years of experience I allow myself to accept the "usual case" and not wrap myself around the specification axle...
Cheers!
You're right. I give up. Might as well just use a hand pump and a picnic tap from now on.Ask yourself this. How many vessels are filled in the supply chain before your tank is actually filled? Are they purging the co2 pump to tank connection 100% before each fill. I 1000% doubt it and if you look at the open ended rig used to fill your tank, a shipload of air is going in first and then the co2. I've watched it firsthand in horror.
Nice straw man.You're right. I give up. Might as well just use a hand pump and a picnic tap from now on.
If we're talking ppm or ppb it's an interesting point. just like the reminder about what's in your tubing when you first connect it.
Any suggestion your beer is ruined, I think we'd both disagree with, but for the sake of thinking how much O2 might be in the tanks I think the refilling method is a valid point.
Actually just a joke. I suppose by now I'd have learned that sarcasm doesn't work on the internet, but sometimes I just can't help myself. Guess I should have used one of them smiley thingies.Nice straw man.
I've read that ppm in the headspace translates to ppb in the beer. Maybe somebody with better math skills than me can show the work.If we're talking ppm or ppb it's an interesting point.
Last edited:
Everything with oxygen and beer is relative. It always has an impact but over a variable amount of time and amount. It can be frustrating to talk about and deal with but the results have been worth it for me.
whattabrau
Well-Known Member
- Joined
- Apr 3, 2021
- Messages
- 116
- Reaction score
- 98
Do you then connect the gas upside down, or how do you prevent the "cup" in the disconnect from instantly being replaced by air? (or are you using some other disconnects besides ball lock?)Very little, I actually purge that. Tank on, ball valve on, press needle nose pliers into the disconnect for a few seconds. Burst that leftover air out (which is theoretically CO2 form the last time because I don't disconnect my lines, but it's PVC so I'm not counting on that for long).
I as a habit press do press the disconnect before connecting it, but with my finger like a caveman. Some of my gas disconnects slowly leak when not connected. Also, it gives me an indication that there is pressure in the gas line, and prevents liquid from contaminating the gas side. So, I think it's a good habit, pliers or not.
I've never purged any other way. Clean keg, throw priming sugar in, hook up to the fermentation, wait some hours (or overnight) until the blowoff container smells like CO2, done. I also sometimes overprime a bit, so that I can spund some liters worth of gas to get rid of those pesky picograms of O2 that snuck into the keg during transfer. (or, some variation of the previous, depending on the beer, how long it will mature, and how long the anticipated consumption time is)I do need to try the fermentation purge.
Look, the answer to your original question is a blindingly obvious "no." If the tables accounted for O2 in the CO2, then they would necessarily show that one can reach a value of zero ppb, ppm, or pp anything. Since the tables do in fact go to zero, they clearly assume pure CO2. Whatever the actual concentration of contaminating O2 in the CO2 used to purge is obviously the minimal concentration of O2 after purging. I guess it was wrong of me (and perhaps others) to jump to the conclusion that you must have realized that and therefore must have been asking something more/else.
Last edited by a moderator:
The trick is in the definition of "ppx." For gases, it's the partial pressure of the gas (or parts per x by number of molecules, same thing.) So if the total CO2 pressure is 2 atm and you have 1 ppm O2, you have a partial pressure for O2 of 2e-6 atm. On the other hand, ppx in liquids is measured by mass, so 1 ppm O2 means 1 mg of O2 per kg of beer.I've read that ppm in he headspace translates to ppb in the beer. Maybe somebody with better math skills than me can show the work.
The Henry's Law constant for O2 at 25 C is 1.3e-3 mol/L atm. So at equilibrium, 2e-6 atm of O2 will give you a concentration of 2.6 nM, ~80 ng/kg, or 80 ppt. 50 ppm O2 in your tank will give you a whopping 4 ppb in your beer. On top of this, it takes time to even get to equilibrium; gases dissolve and diffuse slowly. Consider the days/weeks it takes to force-carbonate using a set-and-forget approach.
However, things are much worse than this. The scenario brewers should consider is that oxygen dissolves into the beer and reacts, which means more oxygen can dissolve, which also reacts ... until all of the oxygen is used up. So the thing to do is to determine the total mass of oxygen the beer is exposed to, and from that calculate what the concentration in the beer would be if all of that oxygen were dissolved.
If you're force-carbonating your beer, the headspace volume doesn't matter as much as the volumes of CO2 used to force. Normal carbonation levels mean 1 L of beer needs 2.5 L of gas (at atmospheric pressure). If that gas were 100% O2, it would have a density of ~1.2 g/L, giving you 3 g of O2 total. If you're at 1 ppm, this means that over the course of force carbonation, you've exposed your beer to 3 ug of O2 ... and 3 ug of O2 per liter of beer is ~3 ug/kg, or 3 ppb.
If your CO2 is ~50 ppm, this means 150 ppb O2 in your beer, which is not a deal-breaker, but it is not negligible by any means. It's certainly worth thinking through the implications -- can the oxygen exposure be lowered, will the beer have a short shelf life, etc.
If you naturally carbonate your beer (spunding or keg conditioning) you can lower the amount of gas the beer is exposed to. Now the only CO2 coming from the tank is what you push into your headspace for serving, so the ratio of headspace volume to beer volume matters. The math is very favorable with a full keg and 10s of mL of headspace, but less so with a near-empty keg and 10 L of headspace.
Let's say 1 L of headspace and 20 L of beer. Let's say you're serving at 2 atm absolute pressure and 50 F, where if it were 100% O2 the density would be ~2.5 g/L. So at 1 ppm, you've got 2.5 ug O2 in the headspace, and 2.5 ug in 20 L of beer is ~0.1 ppb. With 50 ppm in your tank, that's 5 ppb for your beer.
However, when your keg is half empty, you've now got 25 ug of O2 in that 10 L headspace, and it needs to dissolve in only 10 L of beer, so 2.5 ppb. With 50 ppm in your tank, your beer is now at 125 ppb, which again is about where you start to think hard about things.
(Things are worse if there's effective mixing of the gas in your keg with the gas in your tank -- that is, as oxygen dissolves in your beer, more oxygen from the tank comes into the headspace. My gut feeling is that this is going to be very slow and can be ignored.)
TL; DR:
- If you're force-carbonating, typical oxygen levels in your CO2 tank probably do matter. With 50 ppm O2 in the tank, you're looking at ~150 ppb in the keg. This is in the ballpark of current practice for professional breweries, so you should probably expect ok but not stellar results: shelf life measured in weeks or months.
- If you spund (or keg condition), 50 ppm O2 in your CO2 supply will have a negligible impact when your keg is nearly full, but will become more important as you dispense. When half-full, you again have ~125 ppb in the keg.
Similar threads
- Replies
- 2
- Views
- 401
- Replies
- 14
- Views
- 905

