I'm trying to get a good hefeweizen water profile. I'm assuming Hoegaarden is the closest I'm gonna get on the Bru N Water program. I'm trying to get my calcium level to about 90 . As I add Gypsum the chloride starts getting too high. I noticed when I added chalk CaCO3 the calcium went up and the chloride and sulfate stayed in the desired range. I've never used Gypsum and Chalk in combination before . Is this ok or should I use one or the other ? Thanks guys
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Gypsum and chalk
- Thread starter Jag75
- Start date

Help Support Homebrew Talk - Beer, Wine, Mead, & Cider Brewing Discussion Forum:
This site may earn a commission from merchant affiliate
links, including eBay, Amazon, and others.
Gnomebrewer
Well-Known Member
Gypsum doesn't raise chloride - it's Calcium sulphate. I'd strongly suggest you throw those profiles out the window anyway - they're close to useless. I wouldn't use chalk at all in a hefe - there's no need to add alkalinity. IMO, your best German styles is to follow Kai's (braukaiser) water recipes.
http://braukaiser.com/wiki/index.php/Various_water_recipes
http://braukaiser.com/wiki/index.php/Various_water_recipes
Without the chalk addition I have the following.
Calcium 54
Sodium 31
Sulfate 75
Chloride 58
I looked at the link you posted and it had the calcium at 22 . Doesnt a low calcium level hinder yeast during fermentation?
I'm using RO water
Calcium 54
Sodium 31
Sulfate 75
Chloride 58
I looked at the link you posted and it had the calcium at 22 . Doesnt a low calcium level hinder yeast during fermentation?
I'm using RO water
Robert65
Major Obvious (recently promoted)
Chalk cannot be dissolved under any conditions encountered in brewing (this requires extremely low pH and/or extremely high partial pressure of CO2,) and so it contributes nothing at all. Chalk should never be used in brewing. If you need to add alkalinity, you can use either sodium bicarbonate (baking soda) or calcium hydroxide (pickling lime.)
Gnomebrewer
Well-Known Member
I looked at the link you posted and it had the calcium at 22 . Doesnt a low calcium level hinder yeast during fermentation?
Not exactly. I've read several studies on Calcium in beer, and they all seem to have different results. From what I can make out, it's very strain dependent and not completely understood. In general though, it seems lager yeasts don't want too much of it and ale yeasts benefit from moderate to high levels of it for flocculation. You don't normally want hefe yeast to flocculate, so don't need high levels of Calcium. Personally, I don't add any Calcium salts to my water for hefe (rainwater, so similar to RO); I do like a bit of good old table salt in there though.
RPh_Guy
Bringing Sour Back
Besides buffering Lactobacillus starters.Chalk should never be used in brewing.
Would you mind linking to the sources?I've read several studies on Calcium in beer, and they all seem to have different results.
My understanding is that calcium may affect flocculation, and not much else.
Gnomebrewer
Well-Known Member
Would you mind linking to the sources?
My understanding is that calcium may affect flocculation, and not much else.
It was a few years ago that I did a bit of reading about it and I don't have the sources. To clarify, when I said 'different results', I was meaning different results in terms of how Calcium effects flocculation. I haven't read anything saying Calcium is needed for anything else (beyond what is provided by malt - yeast do need some Calcium as do most (all???) living things). So, yes, my understanding is also that added calcium in brewing water only really affects flocculation. The only other interesting thing I remember reading about is how a high Calcium to Magnesium ratio can lead to yeast issues, so when adding Calcium, brewers should also add Magnesium. I don't add Magnesium and do add Calcium to most ales though and my beers taste good to me....
Besides buffering Lactobacillus starters.
My understanding is that calcium may affect flocculation, and not much else.
That's almost the whole story. Yes, it is important for flocculation in beers that need to clear quickly (ales). But the other thing it helps is the longevity of enzymes in the mash. Malt provides ALL the calcium that yeast need for their metabolism. Brewing with calcium-free water is OK, but not necessarily a good idea.
Robert65
Major Obvious (recently promoted)
Oxalate precipitation too.
killian
Well-Known Member
calcium chloride?
McKnuckle
Well-Known Member
I have brewed two very nice weissbiers with only calcium chloride at 0.6g/gal, and no other salts, added to distilled/RO water. Ca = 42, Cl = 74, other ions effectively zero. Mash was adjusted to 5.45 pH with acidulated malt.
RPh_Guy
Bringing Sour Back
@mabrungard & @Robert65
Thank you.
Calcium chloride, anhydrous: 0.45 g/gal
Calcium sulfate: 0.3 g/gal
Sodium chloride: 0.2 g/gal
This gives:
Calcium: 51 ppm
Chloride: 89 ppm
Sulfate: 44 ppm
Sodium: 21 ppm
Chloride and sodium will both enhance the flavor and help provide a full body, putting the malt and yeast forward.
Thank you.
You're never going to get a consensus because this is all based largely on personal preference, but here's my suggestion:I'm trying to get a good hefeweizen water profile.
Calcium chloride, anhydrous: 0.45 g/gal
Calcium sulfate: 0.3 g/gal
Sodium chloride: 0.2 g/gal
This gives:
Calcium: 51 ppm
Chloride: 89 ppm
Sulfate: 44 ppm
Sodium: 21 ppm
Chloride and sodium will both enhance the flavor and help provide a full body, putting the malt and yeast forward.
Gnomebrewer
Well-Known Member
I've seen this in lots of places, including 'How to Brew', but can't find a study to back it up (if you have it, I'd love to read it). I'm not doubting it's true, but would like to know to what extent Calcium effects enzymes, whether it's pH dependent, whether the timeframe is outside what brewers use, which enzymes are affected etc. etc. Without seeing the study, it's quite a vague statement. I'm wondering if it was more of an issue with less modified malts, and doesn't really matter with modern malts and mash schedules. I have three beer recipes that I've brewed several times each that don't use any Calcium additions that get the same efficiency as beers with Calcium added, so my personal experience is that Ca++ doesn't matter in the mash in terms of conversion.But the other thing it helps is the longevity of enzymes in the mash.
RPh_Guy
Bringing Sour Back
I can do research! Take a look through what I found... Pardon the disorganization; I just dumped it all on there.I've seen this in lots of places, including 'How to Brew', but can't find a study to back it up (if you have it, I'd love to read it).
https://***************.com/wiki/Calcium
Feel free to make changes.
Last edited:
Gnomebrewer
Well-Known Member
I can do research! Take a look through what I found... Pardon the disorganization; I just dumped it all on there.
https://***************.com/wiki/Calcium
Feel free to make changes.
Interesting reading, thanks!
It all seems to come back to Bertoft, E., et al. "EFFECT OF pH, TEMPERATURE, AND CALCIUM IONS ON BARLEY MALTct-MYLASE ISOENZYMES." J. hist. Brew. September-October, 1984, Vol. 90, pp. 298-302
(Number 7 on your reference list).
Actually, it comes down to one little snippet out of the whole thing (which I've pasted below for other if they're interested) showing that a-Amylase 1 (Beta amylase???) is stabilised in the presence of CaCl2 at 70C. a-Amylase 2 (Alpha amylase???) isn't affected nearly as much but is inherently more stable at this temperature. It's interesting therefore that other references to this article (eg. number 5 on your reference list) say that Ca++ is needed to stabilise alpha amylase. Or am I misreading the article? Or misinterpreting what amylase 1 and 2 are? The only other reference to Calcium that I can see in the article is how it stabilises enzymes in the presence of Mercury chloride - hardly relevant to brewing. Also, the whole study was based on isolated enzymes added to solutions, not using wort or mash liquor which may provide more of a stabilising environment.
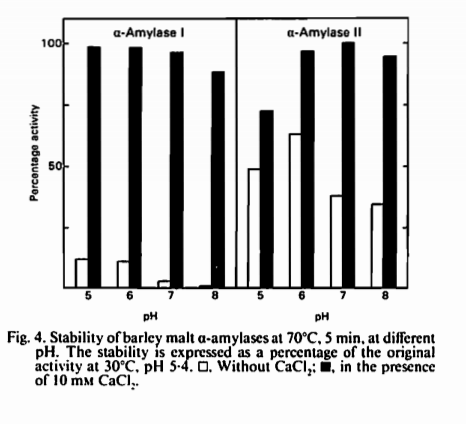
Gnomebrewer
Well-Known Member
I've just re-read the article from above and realised it's only talking about alpha amylase (two isomers), not beta at all.
Similar threads
- Replies
- 42
- Views
- 2K
