scubahiker
Active Member
- Joined
- Oct 21, 2015
- Messages
- 30
- Reaction score
- 3
Hello all! I'm wanting to get into changing my water profile. My local county provided me the water break down for my area:
Calcium (ppm): 8
Magnesium (ppm): N/A
Total Alkalinity as CaCO3: 40.9
Sulfate (ppm): 38.2
Chloride (ppm): 12.9
Sodium (ppm): 35.8
Water pH: 7.8
So I took that into BeerSmiths water profile tool for my base water. Since BeerSmith doesn't take CaCO3, I used bru'n waters calculator to get HCO3 of 49.6. From there I found and tweaked Randy Moshers profile for pale ales. This is what I ended up with:
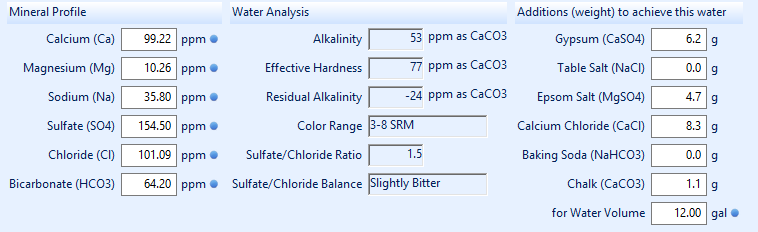
This is for a pale ale. Please let me know your thoughts, as this is all new to me I'm very much open to tweaking!
Calcium (ppm): 8
Magnesium (ppm): N/A
Total Alkalinity as CaCO3: 40.9
Sulfate (ppm): 38.2
Chloride (ppm): 12.9
Sodium (ppm): 35.8
Water pH: 7.8
So I took that into BeerSmiths water profile tool for my base water. Since BeerSmith doesn't take CaCO3, I used bru'n waters calculator to get HCO3 of 49.6. From there I found and tweaked Randy Moshers profile for pale ales. This is what I ended up with:

This is for a pale ale. Please let me know your thoughts, as this is all new to me I'm very much open to tweaking!


