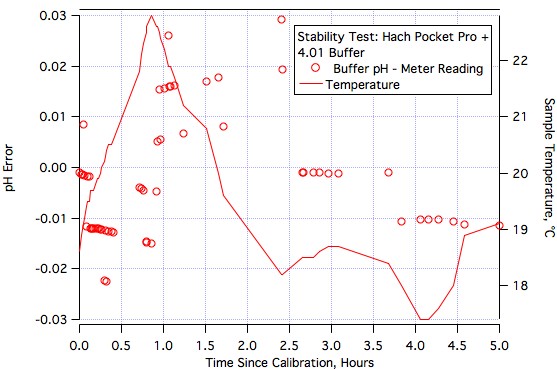
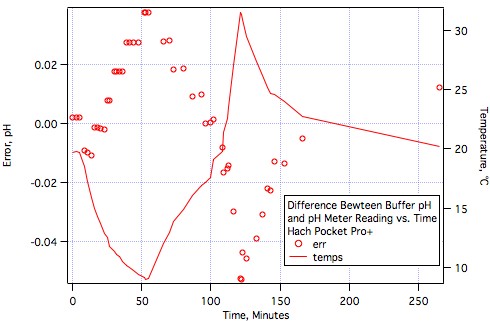
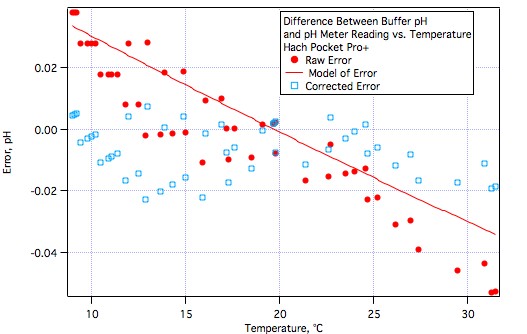
FatsSchindee
Well-Known Member
Hey all,
My next batch will be my first all-grain brew (a Simcoe hop-bursted APA, BIAB), and have been reading up about water chemistry. I have always used RO from the store (at $0.27/gal from the machine) for my extract and PM beers, and want to continue using it for all-grain. I just figure it would be easier to build up from scratch than to figure out the composition of, filter and/or Campden, and then adjust, my tap water. I don't brew more than once a month, and smaller batches, so the cost is negligible to me. I read the primer sticky, and do like the idea of just keeping it simple (Gypsum and CaCl). I'm thinking even simpler, though, as it looks like I may not need any acidulated malt(?). I'm using brewersfriend for the recipe calc, and the associated water calc for the adjustments. It's showing that if I add 5 g gypsum and 3.5 g CaCl (and no acid malt), my mash pH will be 5.25 - right about where I'd want it, no?
Here are the specifics, so you can analyze something I may be missing (and to add to that, I haven't yet used a spreadsheet like Bru'n or Palmer's to double check the numbers, as I'm traveling with only my iPad at the moment, and don't have a spreadsheet app - so maybe those will calculate a different mash pH?):
3 gal batch (post-boil; to end up with at least 2.5 after kettle and ferm trub loss)... So 4.37 gal total (3 mash and 1.37 sparge - BIAB) water to be treated. 6 lbs of grain makes for a 2.0 qt/lb mash thickness. Grains are:
4.75 lbs pale ale malt 3.5L
10 oz light Munich malt 10L
6 oz Crystal40 40L
4 oz Victory 28L
Total IBU of ~44 from 3 oz Simcoe staggered (hop-bursted) from 15 min to flameout
Starting with RO water and adding 5 g gypsum (1.2 tsp) and 3.5 g CaCl (0.8 tsp) gives the following numbers:
Ca - 128
Mg - 0
Na - 0
Cl - 102.1
SO4 - 168.6
SO4/Cl ratio - 1.7
Alkalinity (as CaCO3) - 2.6
RA (as CaCO3) - -88.7
Effective water RA - -132.63
Grist DI water pH - 5.57
Mash pH - 5.25
Do those numbers look good for an APA? I varied from the 1 tsp/5 gal rec from the sticky just a bit by adding more gypsum (the 0.8 tsp would be about right for 4 gal water), as I want (I think... Based on some things I've read; though I've read differing opinions on everything, it seems, which makes this all somewhat confusing) the hops to stand out, and have read a higher SO4/Cl ratio does that (also since I'm not doing a standard early bittering charge, I want to make sure and accentuate the bitterness I do get from the late hops). I've also read that Ca shouldn't be much above 150, and that negligible Mg and Na are okay (get some from grain).
Because I want to keep it simple, I'm not springing for a pH meter yet. I ordered some 4-7 colorpHast strips, and will use those to monitor mash pH (I did read about them reading ~0.3 low, and will try to account for that). If my first few attempts at water modification end up screwing up the taste of my beer, then I'll feel justified buying the meter later. I think I'll buy some Phosphoric acid (seems to be better than Lactic because less flavor?) to have on hand, just in case the pH does measure too high in the mash (after 15-20 min to equalize, I understand). So that'll be my back up instead of the acid malt.
I've been reading up on the subject for the last few weeks (mainly on here, and some other blogs/sites online), and sometimes I feel like I have a decent grasp of the basics... But then I'll revisit part of it another day and realize I didn't understand it quite like I thought I did. Definitely a lot to digest! I've seen it said many times that water chemistry can be as easier or as hard as you make it... So I'm trying to make it as easy as possible for me for this first time! I appreciate any feedback and help I can get... Cheers!
My next batch will be my first all-grain brew (a Simcoe hop-bursted APA, BIAB), and have been reading up about water chemistry. I have always used RO from the store (at $0.27/gal from the machine) for my extract and PM beers, and want to continue using it for all-grain. I just figure it would be easier to build up from scratch than to figure out the composition of, filter and/or Campden, and then adjust, my tap water. I don't brew more than once a month, and smaller batches, so the cost is negligible to me. I read the primer sticky, and do like the idea of just keeping it simple (Gypsum and CaCl). I'm thinking even simpler, though, as it looks like I may not need any acidulated malt(?). I'm using brewersfriend for the recipe calc, and the associated water calc for the adjustments. It's showing that if I add 5 g gypsum and 3.5 g CaCl (and no acid malt), my mash pH will be 5.25 - right about where I'd want it, no?
Here are the specifics, so you can analyze something I may be missing (and to add to that, I haven't yet used a spreadsheet like Bru'n or Palmer's to double check the numbers, as I'm traveling with only my iPad at the moment, and don't have a spreadsheet app - so maybe those will calculate a different mash pH?):
3 gal batch (post-boil; to end up with at least 2.5 after kettle and ferm trub loss)... So 4.37 gal total (3 mash and 1.37 sparge - BIAB) water to be treated. 6 lbs of grain makes for a 2.0 qt/lb mash thickness. Grains are:
4.75 lbs pale ale malt 3.5L
10 oz light Munich malt 10L
6 oz Crystal40 40L
4 oz Victory 28L
Total IBU of ~44 from 3 oz Simcoe staggered (hop-bursted) from 15 min to flameout
Starting with RO water and adding 5 g gypsum (1.2 tsp) and 3.5 g CaCl (0.8 tsp) gives the following numbers:
Ca - 128
Mg - 0
Na - 0
Cl - 102.1
SO4 - 168.6
SO4/Cl ratio - 1.7
Alkalinity (as CaCO3) - 2.6
RA (as CaCO3) - -88.7
Effective water RA - -132.63
Grist DI water pH - 5.57
Mash pH - 5.25
Do those numbers look good for an APA? I varied from the 1 tsp/5 gal rec from the sticky just a bit by adding more gypsum (the 0.8 tsp would be about right for 4 gal water), as I want (I think... Based on some things I've read; though I've read differing opinions on everything, it seems, which makes this all somewhat confusing) the hops to stand out, and have read a higher SO4/Cl ratio does that (also since I'm not doing a standard early bittering charge, I want to make sure and accentuate the bitterness I do get from the late hops). I've also read that Ca shouldn't be much above 150, and that negligible Mg and Na are okay (get some from grain).
Because I want to keep it simple, I'm not springing for a pH meter yet. I ordered some 4-7 colorpHast strips, and will use those to monitor mash pH (I did read about them reading ~0.3 low, and will try to account for that). If my first few attempts at water modification end up screwing up the taste of my beer, then I'll feel justified buying the meter later. I think I'll buy some Phosphoric acid (seems to be better than Lactic because less flavor?) to have on hand, just in case the pH does measure too high in the mash (after 15-20 min to equalize, I understand). So that'll be my back up instead of the acid malt.
I've been reading up on the subject for the last few weeks (mainly on here, and some other blogs/sites online), and sometimes I feel like I have a decent grasp of the basics... But then I'll revisit part of it another day and realize I didn't understand it quite like I thought I did. Definitely a lot to digest! I've seen it said many times that water chemistry can be as easier or as hard as you make it... So I'm trying to make it as easy as possible for me for this first time! I appreciate any feedback and help I can get... Cheers!