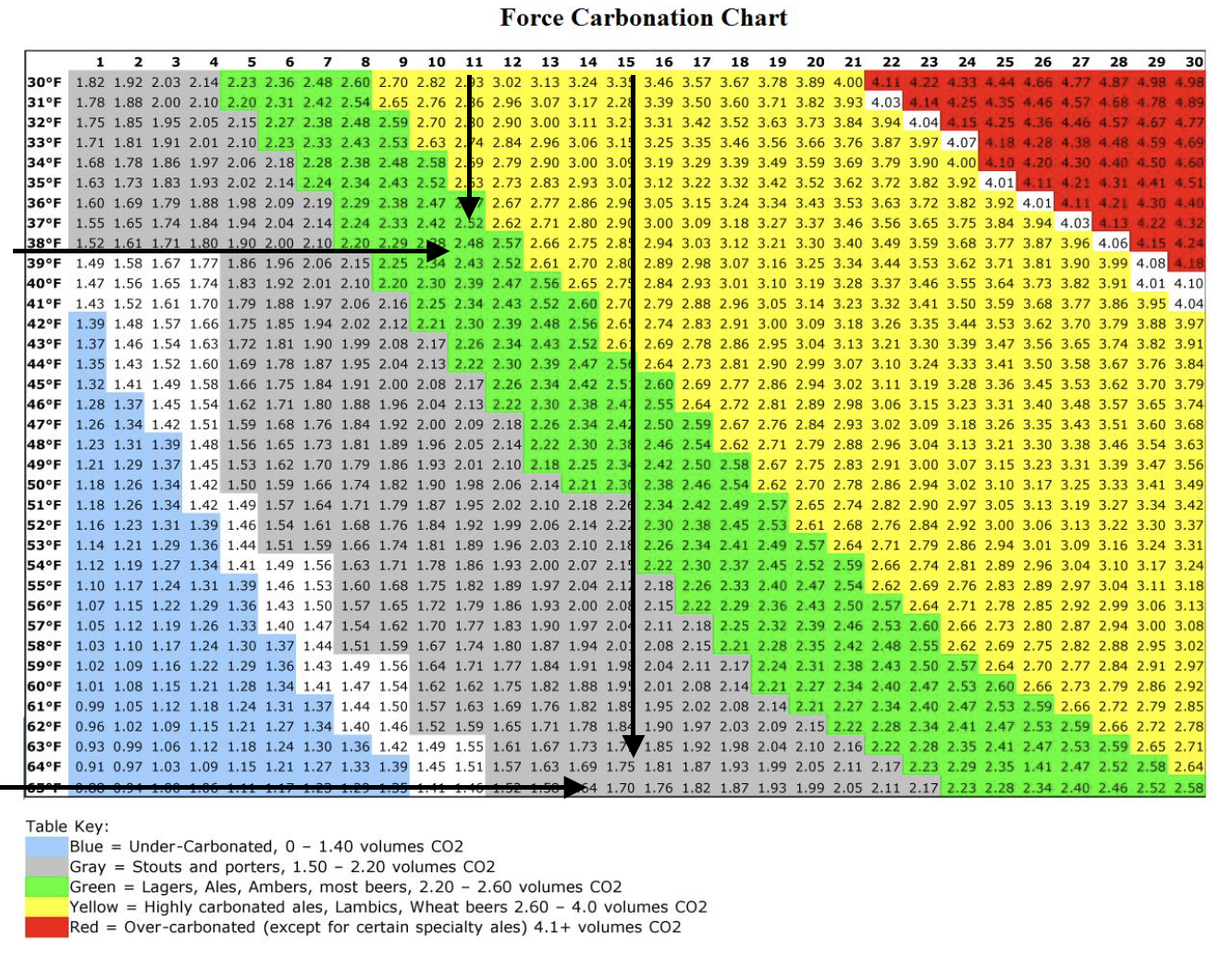
Assume room temperature 65F. SG has dropped to within 5 points of predicted Final Gravity. A spunding valve is attached to the fermenter, set to release at 15 psig. Fermentation continues at 65F and pressure rises inside the sealed fermenter until reaching 15 psig when the spunding valve begins to vent CO2, maintaining the internal 15 psig pressure. Due to the increased partial pressure within the fermenter, CO2 is forced into solution in the beer until the liquid becomes saturated. The chart above indicates that the volume of CO2 contained in the liquid is 1.70 volumes.
At Final Gravity when equilibrium is reached, the saturated liquid (beer) is transferred under 15 psig pressure to a CO2 purged, sealed keg via the black Liquid Out post. A keg mounted spunding valve is placed on the gray Gas In post, with a release pressure set to 'something less' than 15 psig. 65F beer (saturated with CO2 @15 psi) flows into the keg. When the transfer is complete, we nominally have 5 gallons of beer at 65F at 15 psig that contains 1.70 volumes of CO2. There are variables of course, including the dimensions and geometry of "sending" fermenter and the "receiving" keg, the pressure differential between the two of the vessels, the headspace between the beer column and the keg lid, the presence of any residual sugar or non-dormant yeast that slipped from the fermenter to the keg, etc., etc.,
ad infinitum. As you said, the math is complicated and is above my pay grade as well. But I think we can agree that we have at least, nominally, 65F beer saturated with CO2 amounting to 1.70 volumes under 15 psi differential pressure in a sealed keg.
Now we change one variable in the equation. We place the sealed keg in a 38F refrigerator to let it condition for a week or two, even though temperature equilibrium will be reached much sooner. After two weeks we measure the pressure in the keg and find that it has fallen to 11 psig. Does that mean that 4 psi worth of CO2 escaped? No. It went into solution under 15 psig pressure until saturation and equilibration were achieved at 11 psig. The chart above indicates that the volume of saturated CO2 in the beer at that temperature and pressure is 2.48 volumes. The "additional" CO2 wasn't created in a vacuum. It was absorbed into the liquid under pressure from gas in the headspace in a sealed vessel when the temperature was reduced and the liquid was able to hold more of the gas before a new saturation level was reached.
So when I switch the empty keg in my kegerator with a freshly conditioned or lagered keg from my beer fridge, it's usually at 11 psi, which coincidentally is the regulator pressure set point for my kegerator, balanced to deliver beer to the tap at the proper level of carbonation. The kegerator is also set to 38F. The beer is always properly carbonated, even on the first pull.
When I first started kegging nearly 20 years ago (Gawd, am I really getting this old?) I used force carbonation rather than bottle priming. For the longest time I used the "shake and bake/crash and carb" method to get the fizz in. The results were usually random and mixed, but usually over- or under-carbed so I switched to "set and forget" process which took longer but provided consistent, repeatable results. If I wanted an IPA carbonated to 2.5 volumes with a rocky 1½" head that lingered like Belgian Lace leaving notches on the side of the glass marking each sip, I'd hook up the uncarbed keg to 11 psi CO2 and put it in the beer fridge for a week or so. Now I just cut out the middleman, as well as an extra week or two of time, by spunding and cold crashing in a unitank before conditioning or lagering.
What I've outlined is the process I have used for at least my last 10-12 brew sessions since I started using a unitank. My experience has been repeatable and is verifiable based on my brew notes. I'll concede that I don't have scientifically measured empirical data able to pass peer reviewed scrutiny, nor the background or credentials to claim unassailable knowledge of the underlying physics, but these are things I've observed and recorded using the rudimentary tools at my disposal. But that's kinda' the whole reason home brewing exists: to gain more insight, share ideas, improve our methods and brew better (no, GREAT!) beer. Cheers!
Brooo Brother
An Old Dog always wanting and willing to learn New Tricks