I started brewing with filtered tap water and made pretty good beer. I collected the water the day before so the chlorine could off gas, but later started using campden tablets. Wanting to up my game, I ordered a water report from Ward Labs. I started playing with the water calculators and found that I could improve my water for pale ales and IPAs by adding 4 grams of gypsum and 4 grams of calcium chloride. This water profile was also creating good beer. Then I read that all beer needs to have acid added to the mash water, so I went back to the water calculator and played around with the 88% lactic acid numbers. The last beer I brewed was a Belgian IPA using 4 grams of gypsum and calcium chloride and 4ml of 88% lactic acid. This turned out to be one of the worst beers I have made in a long time. My brew session went well so I tend to think the lactic acid messed it up. Do I really need to add acid to my mash water and would 4ml of lactic acid have a big enough impact on flavor to ruin a #5 batch of beer?
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Acid in Mash
- Thread starter wsmith1625
- Start date

Help Support Homebrew Talk - Beer, Wine, Mead, & Cider Brewing Discussion Forum:
This site may earn a commission from merchant affiliate
links, including eBay, Amazon, and others.
4ml of lactic acid in a 5g batch is well below the taste threshold.
What did your calculator say about your mash pH before the addition of acid? Just the salts may have been enough to get the pH in a favorable range.
In some of my recipes I don't need acid to lower the pH as the salts alone are sufficient, granted I brew mostly Brittish styles and adjust my water to UK levels of mineral content.
What did your calculator say about your mash pH before the addition of acid? Just the salts may have been enough to get the pH in a favorable range.
In some of my recipes I don't need acid to lower the pH as the salts alone are sufficient, granted I brew mostly Brittish styles and adjust my water to UK levels of mineral content.
Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
Where did you read that? Many beers do not require an addition of an acid, and some require the addition of Baking Soda, the opposite of an acid.
I agree with Eric that 4 mL of 88% Lactic Acid is not enough to induce a noticeable negative flavor perception.
I agree with Eric that 4 mL of 88% Lactic Acid is not enough to induce a noticeable negative flavor perception.
I used the advanced calculator on BrewersFriend with the light and hoppy profile. My numbers looked okay with the lactic acid. It was a new recipe for me so something else could have caused it to taste bad. It was a Houblon Chouffe clone and tasted nothing like the real thing. Not even enjoyable really. Everything was new to me in that brew, my first high gravity beer, my first multi step starter, first time using the yeast strain, and first time using lactic acid. I'm try the recipe again and see if I do better, but really wanted to rule out the lactic acid as the problem.4ml of lactic acid in a 5g batch is well below the taste threshold.
What did your calculator say about your mash pH before the addition of acid? Just the salts may have been enough to get the pH in a favorable range.
In some of my recipes I don't need acid to lower the pH as the salts alone are sufficient, granted I brew mostly Brittish styles and adjust my water to UK levels of mineral content.
How old is the beer? It could just be that you have been too impatient, these type of beers often need 6+ months to really shine...
It was a thread on HBT. I don't recall who posted it, but they said that all beer needs some sort of acid added to the mash and it was not disputed. Also, Houblon who posted the recipe said he played with lactic acid for his water profile. Honestly my water test is old and things could have changed. I don't have a PH meter which I know I should have, but everything I read about them makes me want to avoid then. Basically when to take the reading, when to adjust with acid, and the accuracy of the meters ask sound discouraging to me. There's no right answer because if you check at 15 minutes most conversion is done already. I would rather use the software and hope I'm close.Where did you read that? Many beers do not require an addition of an acid, and some require the addition of Baking Soda, the opposite of an acid.
I agree with Eric that 4 mL of 88% Lactic Acid is not enough to induce a noticeable negative flavor perception.
It's pretty young, 7 weeks maybe. The recipe calls for 2 weeks to ferment, dry hop and lager for 3 weeks, then carb and serve.How old is the beer? It could just be that you have been too impatient, these type of beers often need 6+ months to really shine...
Hm, then I don't know what might have gone wrong.
What sort of off flavours? Could it be related to some fermentation issue?
What sort of off flavours? Could it be related to some fermentation issue?
Could have been. I pitched at 75 and let it rise to 78, only to read the recipe a 2nd time and saw that he left it at 75. It was Wyeaset 3522 Belgian Ardennes. I did a 2 liter starter and then after 3 days decanted and added another 2 liters to get the cell count up. I expected to maybe get some unwanted banana or clove flavors from the high temp, but they're not really present.
First thought, not exactly related, is that equal amounts of gypsum and calcium chloride aren't normal for an IPA. I am far heavier on the gypsum myself so I can get the chloride / sulfate ratio more hoppy. something like a 4:1 ratio. A 1:1 ratio feels like it's more suited for a stout. You shold do what works for you but maybe think about this.
I add acid but it's 10% acid. And something like 8ml for a 5 gallon IPA. If you're adding 88% acid, even just 4ml, it sounds like quite a pH shift. The guys above know far more than I do but - when you do the calculator be sure you let the calculator know what % acid you are using. If it defaults to 10% and you use 88% then you are probably using far too much. Even if you don't taste it directly I'm not sure you're going to get the pH you are after (if, again, the calculator is set for 10%).
I add acid but it's 10% acid. And something like 8ml for a 5 gallon IPA. If you're adding 88% acid, even just 4ml, it sounds like quite a pH shift. The guys above know far more than I do but - when you do the calculator be sure you let the calculator know what % acid you are using. If it defaults to 10% and you use 88% then you are probably using far too much. Even if you don't taste it directly I'm not sure you're going to get the pH you are after (if, again, the calculator is set for 10%).
My gut tells me it's the lactic acid. I'm going to have to brew this recipe again and omit the acid and change my gypsum to calcium chloride ratio. Good call @tracer bullet . Unfortunately I won't be able to get to it for a while, but when I do I'll try to post my results.
First thought, not exactly related, is that equal amounts of gypsum and calcium chloride aren't normal for an IPA. I am far heavier on the gypsum myself so I can get the chloride / sulfate ratio more hoppy. something like a 4:1 ratio. A 1:1 ratio feels like it's more suited for a stout. You shold do what works for you but maybe think about this.
I add acid but it's 10% acid. And something like 8ml for a 5 gallon IPA. If you're adding 88% acid, even just 4ml, it sounds like quite a pH shift. The guys above know far more than I do but - when you do the calculator be sure you let the calculator know what % acid you are using. If it defaults to 10% and you use 88% then you are probably using far too much. Even if you don't taste it directly I'm not sure you're going to get the pH you are after (if, again, the calculator is set for 10%).
These are great points. The only thing I would say regarding the sulfate/chloride is it depends what the initial water profile is to begin with in regards to whether the additions are equal or not.
I'm assuming this is the profile the OP was targeting.

Last edited:
I'll use the water calculator to get the correct ratio. Omitting the lactic acid and tightening my fermentation temperature down should help a lot.
That's a good profile I've had good IPA's using a similar one in beer smith. It really sounds like an acid issue and dialing in pH with the calculator. 4 mils at 88% is quite a bit of acid.I'll use the water calculator to get the correct ratio. Omitting the lactic acid and tightening my fermentation temperature down should help a lot.
Cheers!
Good point that we all have different water and what works for one isn't right for another.
Also a note on the Ward's lab analysis is to keep an eye on the SO4 results, there's a thing about multiplying SO4-S by 3 for some (maybe all) calculators and it could mess you up as well.
Also a note on the Ward's lab analysis is to keep an eye on the SO4 results, there's a thing about multiplying SO4-S by 3 for some (maybe all) calculators and it could mess you up as well.
It was a thread on HBT. I don't recall who posted it, but they said that all beer needs some sort of acid added to the mash and it was not disputed. Also, Houblon who posted the recipe said he played with lactic acid for his water profile. Honestly my water test is old and things could have changed. I don't have a PH meter which I know I should have, but everything I read about them makes me want to avoid then. Basically when to take the reading, when to adjust with acid, and the accuracy of the meters ask sound discouraging to me. There's no right answer because if you check at 15 minutes most conversion is done already. I would rather use the software and hope I'm close.
What was your predicted mash pH from the software? The amount of acid you add should be based on that, not just adding some to add it.
Is hout's description of your target water profile what you were trying to use?
You don't NEED a pH meter to make good beer, but if you're trying to troubleshoot something, knowing the actual mash pH would help. If your actual mash pH is way off, then your assumptions somewhere are off - maybe source water changed, maybe an ingredient got mis-measured, maybe an ingredient was mislabeled, maybe something was calculated incorrectly.
I don’t know where that came from. As a general statement, acids are not always required. The purpose of adding acids to the mash is to adjust mash ph for optimal enzyme activity.
It depends on your grain bill. Lighter grains and base malts are higher ph. Dark and roasted malts are lower ph. When you’re making pilseners or lagers that are all light grains you will generally need to lower the mash ph with acid. If you’re making a stout with lots of dark grains you might not need any,
Do you use software like Brun’Water or EZ Water calculator? Those have you enter your grain bill and will predict ph. You can enter your minerals and acids and they will show you the changes. I have never used 88% acid. I prefer to buy acid malt and use that because for me its easier and I don’t like the idea of storing a strong acid and having to wear rubber gloves to handle it.
Far as Gypsum and Calcium Chloride - Gypsum adds calcium and sulfate and Calcium Chloride adds calcium and chloride. You got your water report from Ward and thats a great place to start, knowing where your numbers are. People do ratios of chloride to sulfate. Having more chloride than sulfate will result in a “fuller” beer that people say accentuates maltiness. Having more sulfate than chloride results in a “drier” beer that people say accentuates hoppiness.
You can raise your levels by adding minerals. The only way to really lower levels of any minerals is to dillute your water with distilled water or RO water.
Some people pay alot of attention to city water profiles and try to emulate water from other cities where beers are brewed, such as Pilsen or Burton on Trent. I’ve never been a fan of that because brewers in those cities are adjusting their water too. So how do you know what they are doing? Adjusting to their water profile is not the be all and end all.
These are some general things. I’m still pretty new to water chemistry myself and I often am still seeking advice from others here too.
It depends on your grain bill. Lighter grains and base malts are higher ph. Dark and roasted malts are lower ph. When you’re making pilseners or lagers that are all light grains you will generally need to lower the mash ph with acid. If you’re making a stout with lots of dark grains you might not need any,
Do you use software like Brun’Water or EZ Water calculator? Those have you enter your grain bill and will predict ph. You can enter your minerals and acids and they will show you the changes. I have never used 88% acid. I prefer to buy acid malt and use that because for me its easier and I don’t like the idea of storing a strong acid and having to wear rubber gloves to handle it.
Far as Gypsum and Calcium Chloride - Gypsum adds calcium and sulfate and Calcium Chloride adds calcium and chloride. You got your water report from Ward and thats a great place to start, knowing where your numbers are. People do ratios of chloride to sulfate. Having more chloride than sulfate will result in a “fuller” beer that people say accentuates maltiness. Having more sulfate than chloride results in a “drier” beer that people say accentuates hoppiness.
You can raise your levels by adding minerals. The only way to really lower levels of any minerals is to dillute your water with distilled water or RO water.
Some people pay alot of attention to city water profiles and try to emulate water from other cities where beers are brewed, such as Pilsen or Burton on Trent. I’ve never been a fan of that because brewers in those cities are adjusting their water too. So how do you know what they are doing? Adjusting to their water profile is not the be all and end all.
These are some general things. I’m still pretty new to water chemistry myself and I often am still seeking advice from others here too.
Last edited:
Actually I found my water profile.
Mash Chemistry and Brewing Water Calculator | Brewer's Friend (brewersfriend.com)
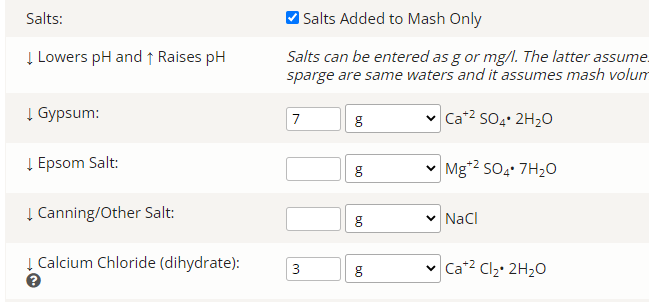
7 grams gypsum
3 grams calcium chloride
4.55 ml lactic acid, but I forgot to enter the concentration percent
Mash Chemistry and Brewing Water Calculator | Brewer's Friend (brewersfriend.com)
7 grams gypsum
3 grams calcium chloride
4.55 ml lactic acid, but I forgot to enter the concentration percent
I don’t know where that came from. As a general statement, acids are not always required. The purpose of adding acids to the mash is to adjust mash ph for optimal enzyme activity.
It depends on your grain bill. Lighter grains and base malts are higher ph. Dark and roasted malts are lower ph. When you’re making pilseners or lagers that are all light grains you will generally need to lower the mash ph with acid. If you’re making a stout with lots of dark grains you might not need any,
Do you use software like Brun’Water or EZ Water calculator? Those have you enter your grain bill and will predict ph. You can enter your minerals and acids and they will show you the changes. I have never used 88% acid. I prefer to buy acid malt and use that because for me its easier and I don’t like the idea of storing a strong acid and having to wear rubber gloves to handle it.
Far as Gypsum and Calcium Chloride - Gypsum adds calcium and sulfate and Calcium Chloride adds calcium and chloride. You got your water report from Ward and thats a great place to start, knowing where your numbers are. People do ratios of chloride to sulfate. Having more chloride than sulfate will result in a “fuller” beer that people say accentuates maltiness. Having more sulfate than chloride results in a “drier” beer that people say accentuates hoppiness.
You can raise you levels by adding minerals. The only way to really lower levels of any minerals is to dillute your water with distilled water or RO water.
Some people pay alot of attention to city water profiles and try to emulate water from other cities where beers are brewed, such as Pilsen or Burton on Trent. I’ve never been a fan of that because brewers in those cities are adjusting their water too. So how do you know what they are doing? Adjusting to their water profile is not the be all and end all.
These are some general things. I’m still pretty new to water chemistry myself and I often am still seeking advice from others here too.
Also Mash Made Easy from @Silver_Is_Money is a great calculator too.
It says Mash pH N/A. Add in the 88% and see what the mash pH comes too.Actually I found my water profile.
Mash Chemistry and Brewing Water Calculator | Brewer's Friend (brewersfriend.com)
7 grams gypsum
3 grams calcium chloride
4.55 ml lactic acid, but I forgot to enter the concentration percent
With the 88% added. Looks good.


Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
Due to "Beer Laws" virtually every brewery in Germany adds Lactic Acid in one form or another to their lighter colored beers, and some add lots of it. If lactic acid was ruining beer, no one told them. Or their satisfied customers worldwide.
I agree about water profiles changing too. I had my water tested here in March of 2019. My results said I have high Chloride and high sodium and almost no sulfate. I’ve been working off that report with decent results. But its got me thinking what if it snowed here and they put salts down on the roads that washed into the aquifers? That would result in, hmmm, high sodium and high chloride. So I plan to get mine tested again now. I’m going to wait til about the middle of June this year and send it in again.
Looks like this takes your grain bill in to account too.With the 88% added. Looks good.
View attachment 766543
I'm not super familiar with brewers friend but your stuff appears to be checking the calculators boxes. Not sure what model they use to predict pH. Somebody like @Silver_Is_Money or @mabrungard would be more fit to help if water chem is the issue.
Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
With Brewer's Friend indicating 'met' perfection from a water perspective, why should anyone be blaming the water? And ditto for the 4 or 4.5 mL of 88% Lactic Acid and the resultant 'nominal' Mash pH of 5.40.
My guess is that there is either a process or yeast related problem, or both. And such matters are best discussed (IMHO) in other sub-fourms.
My guess is that there is either a process or yeast related problem, or both. And such matters are best discussed (IMHO) in other sub-fourms.
I agree that lactic acid was probably ruled out and other contributing factors would be more appropriate in other sub-forums. I am going to brew this recipe again, take better notes, and not use the lactic acid because I don't think it's absolutely necessary. Thanks for all the feedback.With Brewer's Friend indicating 'met' perfection from a water perspective, why should anyone be blaming the water? And ditto for the 4 or 4.5 mL of 88% Lactic Acid and the resultant 'nominal' Mash pH of 5.40.
My guess is that there is either a process or yeast related problem, or both. And such matters are best discussed (IMHO) in other sub-fourms.
Was just going to add another comment against the notion that all beers need acid. Technically there is acidic effect to the water when adding darker malts in the first place so in that light, there's at least some truth to it. But you shouldn't arbitrarily add it.
I only use phosphoric acid when brewing lighter color beers that don't have darker malts to help lower pH naturally and when other minerals cant do it alone. And now that I've started using 100% DI, I haven't had to use any so far.
I only use phosphoric acid when brewing lighter color beers that don't have darker malts to help lower pH naturally and when other minerals cant do it alone. And now that I've started using 100% DI, I haven't had to use any so far.
Just another side note: both Gypsum and Calcium Chloride will lower mash ph also, though not as drastically as acid or acid malt. When you enter your minerals, enter Gypsum and Calcium Chloride first if you need to add those. Then enter any acid or acid malt last to make final ph adjustment. Both of those will also raise calcium which is generally a good thing.Actually I found my water profile.
Mash Chemistry and Brewing Water Calculator | Brewer's Friend (brewersfriend.com)
7 grams gypsum
3 grams calcium chloride
4.55 ml lactic acid, but I forgot to enter the concentration percent
Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
Just another side note: both Gypsum and Calcium Chloride will lower mash ph also, though not as drastically as acid or acid malt. When you enter your minerals, enter Gypsum and Calcium Chloride first if you need to add those. Then enter any acid or acid malt last to make final ph adjustment. Both of those will also raise calcium which is generally a good thing.
Beware that modern research indicates that within the mash "proper" the impact of pH lowering via the addition of Calcium ions into ones mash water is actually measured to range from between 1/2 to 1/4 of that previously believed and dogmatically accepted. Only one mash pH assistant software/spreadsheet offering (that I'm aware of) is capable of being dialed into compliance with the latest research by the user, and is downloaded pre-set to compliance with 1/2. The others still follow antiquated (and effectively research falsified) guidelines with respect to the mash pH lowering via the addition of Calcium.
With Brewer's Friend indicating 'met' perfection from a water perspective, why should anyone be blaming the water? And ditto for the 4 or 4.5 mL of 88% Lactic Acid and the resultant 'nominal' Mash pH of 5.40.
My guess is that there is either a process or yeast related problem, or both. And such matters are best discussed (IMHO) in other sub-fourms.
Because at the time we didn't have that information. Brewer's Friend should be reasonably trustworthy and so, yes, it's looking better now.
Question for you - not arguing just asking - does 4ml at 88% sound normal to you? It may very well be and be fine. It's just, I think, far more than I use. Maybe of course I'm all wrong. 8ml at 10% would be more in line for my IPA's. And it looks like my water isn't so terribly different - not a whole lot of minerals in it.
@wsmith1625 - did you catch the note on the sulfate reading? If you haven't already, be sure that you put the Ward's info into the calculator correctly. It may not be a direct entry of the Ward's result. It won't single handedly be a huge issue but just to be sure things are as accurate as they can be made to be.
If it's been reasonably established the water was OK, - you might describe your off-flavors, maybe folks could send you down a new direction (i.e. yeast).
Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
Question for you - not arguing just asking - does 4ml at 88% sound normal to you?
I could only attempt to 'nominally' (as opposed to specifically) answer your question if I had every pertinent detail as to 'source' water analyticals, mineral additions to same, water volumes (with emphasis upon the mash, but also with respect to the sparge), grain and adjunct details, and grain and adjunct weights.
I could only attempt to 'nominally' (as opposed to specifically) answer your question if I had every pertinent detail as to 'source' water analyticals, mineral additions to same, water volumes (with emphasis upon the mash, but also with respect to the sparge), grain and adjunct details, and grain and adjunct weights.
From the Brewer's Friend link:
No Sparge, 7.5 gallons water

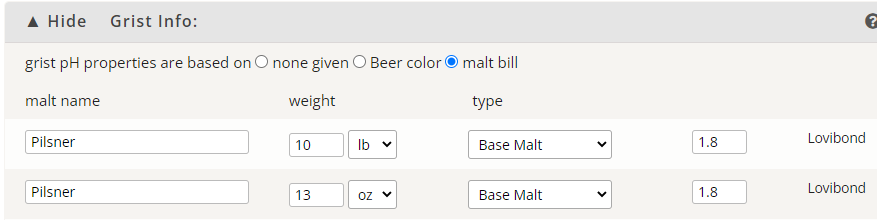
Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
For the presumption of 100% pH reduction due to Calcium within the mash I compute a need for 4.34 mL of 88% Lactic Acid whereby to 'nominally' achieve a mash pH of 5.40.
For the presumption of only 50% pH reduction due to Calcium within the mash I compute a need for 6.25 mL of 88% Lactic Acid whereby to 'nominally' achieve a mash pH of 5.40.
For the presumption of only 50% pH reduction due to Calcium within the mash I compute a need for 6.25 mL of 88% Lactic Acid whereby to 'nominally' achieve a mash pH of 5.40.
Is that yours?Beware that modern research indicates that within the mash "proper" the impact of pH lowering via the addition of Calcium ions into ones mash water is actually measured to range from between 1/2 to 1/4 of that previously believed and dogmatically accepted. Only one mash pH assistant software/spreadsheet offering (that I'm aware of) is capable of being dialed into compliance with the latest research by the user, and is downloaded pre-set to compliance with 1/2. The others still follow antiquated (and effectively research falsified) guidelines with respect to the mash pH lowering via the addition of Calcium.
I’ve been using the EZ Water Calculator. It seemed to have less of a learning curve and fewer instructions than Brun’Water. I also have a mac and software written for Excel sometimes works goofy on a mac. Apple uses a program called Numbers that is similar to Excel but I found its not always 100% compatible. Some cells and formulas and such don’t transfer over intact. I had problems with Brun’Water on the mac. EZ Water calculator works pretty well.
Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
Is that yours?
No, the researchers who reached this conclusion are:
1) Initially AJ deLange said he found the pH reduction due to extant plus added calcium ions within the mash proper to be generally ~1/2 of Kolbach.
2) More recently research Chemists Barth and Zaman found that the pH reduction due to extant plus added calcium ions within the mash proper ranged from ~1/2 to ~1/4 of Kolbach, with the outcome being base malt dependent. And they published a peer reviewed paper to that effect. I have a copy of their paper, but anyone who is interested must pay for access to it so I'm not able to simply upload my copy here.
Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
I’ve been using the EZ Water Calculator. It seemed to have less of a learning curve and fewer instructions than Brun’Water. I also have a mac and software written for Excel sometimes works goofy on a mac. Apple uses a program called Numbers that is similar to Excel but I found its not always 100% compatible. Some cells and formulas and such don’t transfer over intact. I had problems with Brun’Water on the mac. EZ Water calculator works pretty well.
EZ Water is indeed easy to use, but to my knowledge it has not been maintained or upgraded in nearly a decade.
Which package is that just out of curiosity?No, the researchers who reached this conclusion are:
1) Initially AJ deLange said he found the pH reduction due to extant plus added calcium ions within the mash proper to be generally ~1/2 of Kolbach.
2) More recently research Chemists Barth and Zaman found that the pH reduction due to extant plus added calcium ions within the mash proper ranged from ~1/2 to ~1/4 of Kolbach, with the outcome being base malt dependent. And they published a peer reviewed paper to that effect. I have a copy of their paper, but anyone who is interested must pay for access to it so I'm not able to simply upload my copy here.
The types of malt that can be entered leave something to be desired too.EZ Water is indeed easy to use, but to my knowledge it has not been maintained or upgraded in nearly a decade.
Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
Which package is that just out of curiosity?
See the short blurb beneath my avatar.
Similar threads
- Replies
- 42
- Views
- 1K
- Replies
- 9
- Views
- 748

