You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
2 water Reports give me your opinion
- Thread starter BIAB-Tim
- Start date

Help Support Homebrew Talk - Beer, Wine, Mead, & Cider Brewing Discussion Forum:
This site may earn a commission from merchant affiliate
links, including eBay, Amazon, and others.
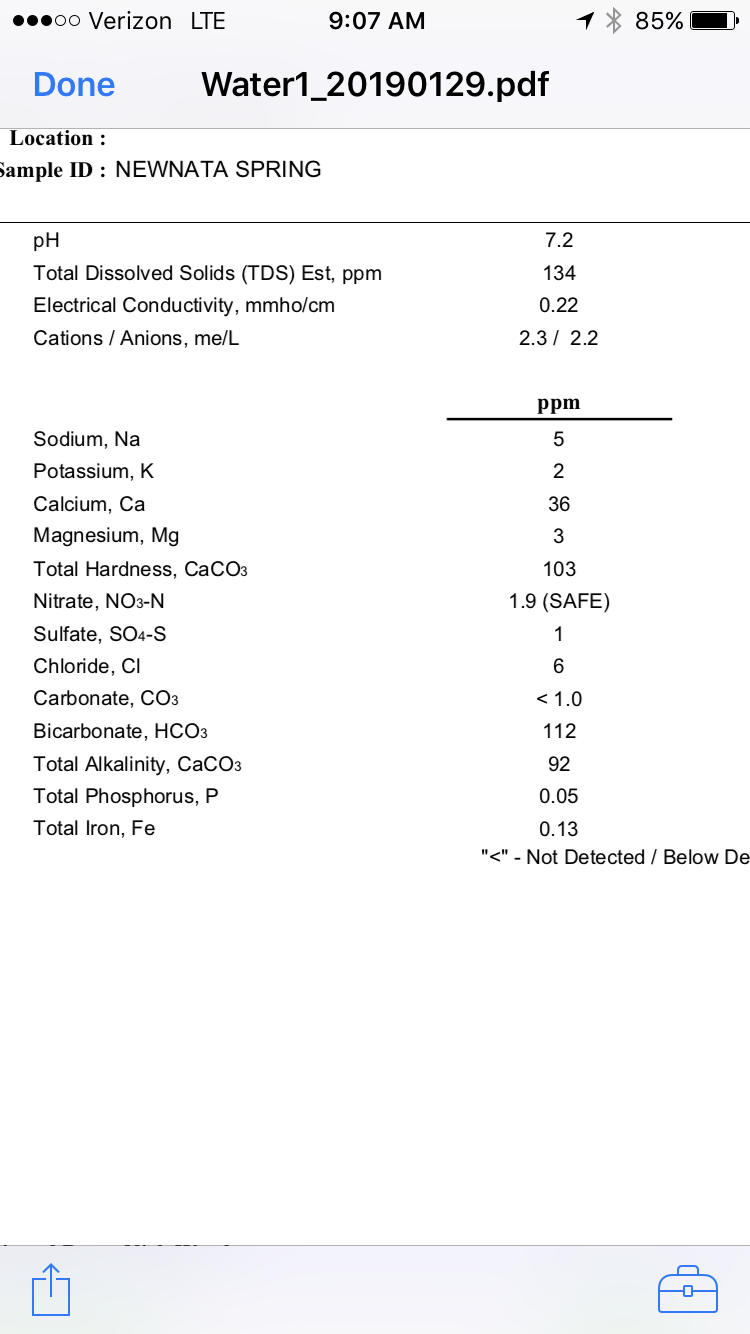
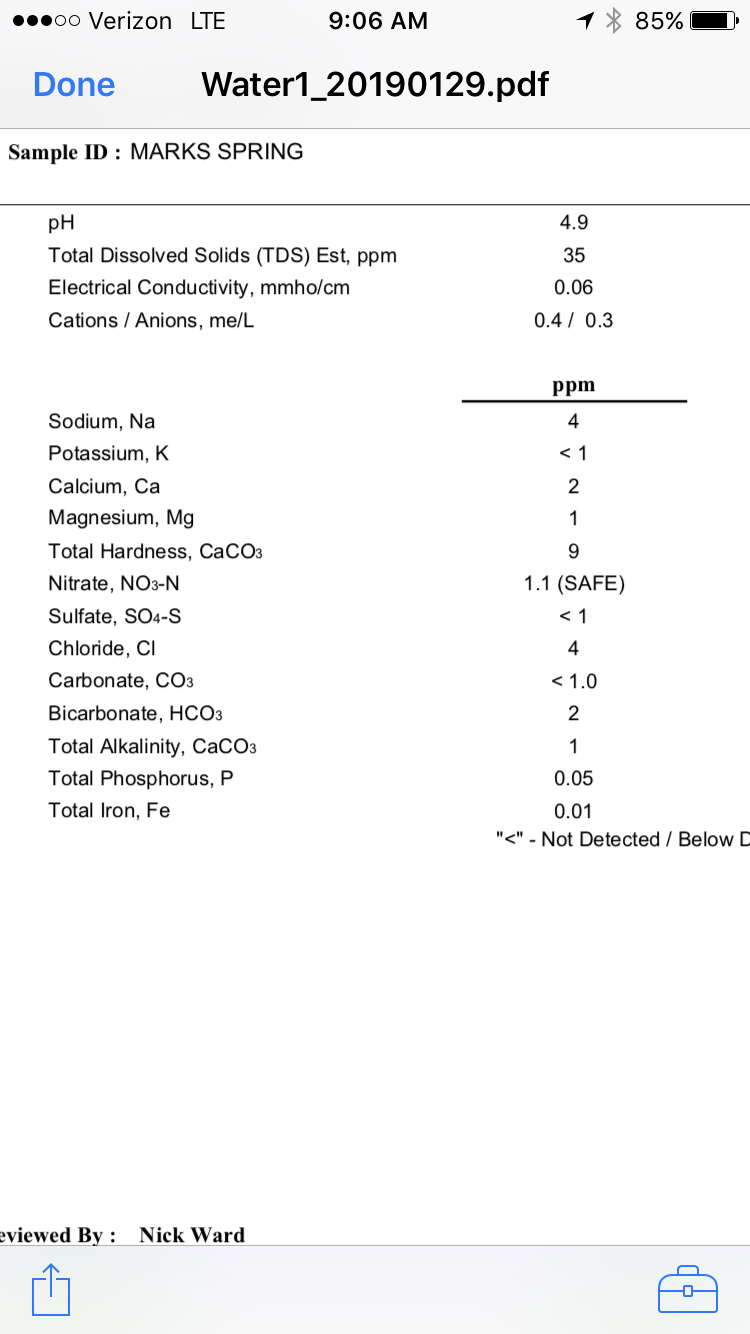
The second one, the mineral content is real low and perfect for building up a water profile per style...however, a pH of 4.9 seems real low and I would be worried about low mash pH since most common minerals used for adjusting water, gypsum, Calcium Chloride and Epsom salt lower the mash pH. You would need chalk or baking soda to increase it. If I had to pick one to use, I would use the first one.
Both water reports are decent to use either source, though I would gravitate towards Mark's Spring as it approaches the content of my RO water and RO water is so easy to work with for virtually any style/recipe.
And don't worry about the pH of raw water as it's invariably too weak to have any practical effect on a mash...
Cheers!
And don't worry about the pH of raw water as it's invariably too weak to have any practical effect on a mash...
Cheers!
What are we looking at? Two lab reports on the same water? The first one is not too off from mine (you have higher Bicarbonate and Total Alkalinity, but less Sodium, Sulfate and Chloride. That second one seem very low, but 4.9 pH seems very acidic.
(I am a total noob when it comes to water chemistry...something I am trying to learn and figure out a plan for my brews)
(I am a total noob when it comes to water chemistry...something I am trying to learn and figure out a plan for my brews)
Not the same, but both very good for brewing.
And again, pH of source water is nearly meaningless as it will be swamped by the grain effects...
Cheers!
And again, pH of source water is nearly meaningless as it will be swamped by the grain effects...
Cheers!
Soulshine2
Well-Known Member
It all depends on the beer to be brewed as to what your starting water characteristics should be. If you're anal about water chemistry , all needs to be done is adjust your starting water, the grains (in the style youre after) will in turn impart their own pH into the final characteristics of the beer produced. Meaning, dont start with a water profile consistent with a Czech Pilsener to make an Irish Stout and expect it to turn out true to style. Water hardness or softness does have an effect , positively or negatively, depends on what your end product is . Do some reading on the history of the beer style youre making and adjust to that. RDWAHAHBNot the same, but both very good for brewing.
And again, pH of source water is nearly meaningless as it will be swamped by the grain effects...
Cheers!
edit- sorry, directed this to the OP.
Lefou
Danged rascally furt
- Joined
- May 1, 2016
- Messages
- 2,300
- Reaction score
- 1,227
I'd choose to brew with water from the first profile and will tell you why.
First off, it's low mineral "soft" water. The alkalinity is very good, pH is good, and the only things I would do for a low SRM beer (Pilsner, blonde, or Helles) would be adding calcium brewing salts after running it through a carbon filter. Very good as a base water to build on.
The second water profile has a moderately low acidic pH, low dissolved solids and very little alkalinity. The water can be balanced and the pH adjusted by adding a portion of CaCO3 and sodium bicarbonate, then boiled. When it cools the excess carbonates that don't bind will precipitate. The relative pH can then be checked with a meter or even a cheap water test from a pool supply shop, then your next step would be to filter and add the desired brewing salts.
First off, it's low mineral "soft" water. The alkalinity is very good, pH is good, and the only things I would do for a low SRM beer (Pilsner, blonde, or Helles) would be adding calcium brewing salts after running it through a carbon filter. Very good as a base water to build on.
The second water profile has a moderately low acidic pH, low dissolved solids and very little alkalinity. The water can be balanced and the pH adjusted by adding a portion of CaCO3 and sodium bicarbonate, then boiled. When it cools the excess carbonates that don't bind will precipitate. The relative pH can then be checked with a meter or even a cheap water test from a pool supply shop, then your next step would be to filter and add the desired brewing salts.
Not following any of that. The water is balanced. If it's sitting in a bottle on his bench it is balanced. If you add sodium bicarbonate to this water it will dissolve. If you add calcium carbonate it won't (to any appreciable extent). If you then boil the water nothing will happen. The bicarbonate will stay in solution and the calcium carbonate as already 'preciptated'. It never dissolved.
To get rid of the alkalinity caused by the addition of bicarbonate one would have to add a calcium salt and then boil. This would precipitate part of the bicarbonate leaving about 1 mEq/L acidity. He's got 0.02 now. Why would he want to spoil that?
The pH reading of 4.9 is probably caused by the fact that the water has so little of anything in it. It is hard to get a good pH meter reading under those circumstances. Even if the reading is correct it has little or no significance with an alkalinity of 0.02 mEq/L. IOW there is no reason to adjust the pH. It is fine as it is. The source water pH frequently has so little effect that many calculators and spread sheets ignore it (though they shouldn't).
To get rid of the alkalinity caused by the addition of bicarbonate one would have to add a calcium salt and then boil. This would precipitate part of the bicarbonate leaving about 1 mEq/L acidity. He's got 0.02 now. Why would he want to spoil that?
The pH reading of 4.9 is probably caused by the fact that the water has so little of anything in it. It is hard to get a good pH meter reading under those circumstances. Even if the reading is correct it has little or no significance with an alkalinity of 0.02 mEq/L. IOW there is no reason to adjust the pH. It is fine as it is. The source water pH frequently has so little effect that many calculators and spread sheets ignore it (though they shouldn't).
Lefou
Danged rascally furt
- Joined
- May 1, 2016
- Messages
- 2,300
- Reaction score
- 1,227
The second water report seems slightly odd to me and pings my radar.
It seems strangely absent of ground water mineral content and looks more like country rain water captured in a basin. I wouldn't use it to brew with until it got filtered AND treated with calcium salts, but that's me.
It seems strangely absent of ground water mineral content and looks more like country rain water captured in a basin. I wouldn't use it to brew with until it got filtered AND treated with calcium salts, but that's me.
Last edited: