lupulinaddict
Well-Known Member
- Joined
- Jul 24, 2014
- Messages
- 486
- Reaction score
- 131
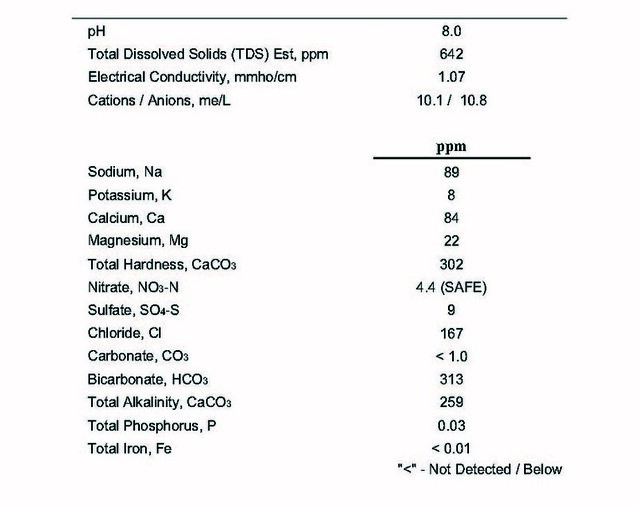
So I have been trying to learn how to work with my water without posting. It seems like when I get a grasp on it, I read a little more, and then I get more confused. If anyone wouldn't mind helping this is where I'm at. It seems like my water has more bicarbonate, calcium, chloride, and sodium than most reports I have been reading on here. I tried using Brewers friend (I have to dl excel on my comp for the others) and I have to add, what seems like to me, a lot of gypsum to get the ph lower. I think since my bicarbonate and calcium is high I should dilute the water first before adding anything? Would that be the first step you guys advise? Also, I read a thread where someone said you can't use your cities report because it's just an average of tests, but the thread died. Can someone explain why that is? It seems like the water would change yearly so an average would be a good starting point if I didn't buy a complete test kit yet. Thanks water gods! View attachment ImageUploadedByHome Brew1457103143.737655.jpg