Hello,
This is my second venture with adjusting my water profile to obtain proper mash pH and flavor profile. I was hoping some of you guys with more info could look this over and let me know if I'm on the right track.
I am planning to steep the Roasted Barley and Chocolate Malt in the boil kettle, to avoid them throwing off my mash pH. I've never done this before, since I've never calculated my pH before. I believe that is pretty common practice for this reason, right?
Thanks!
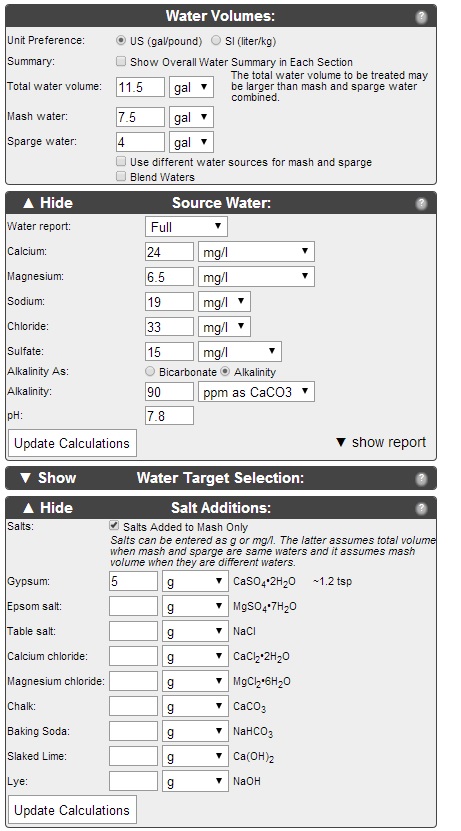
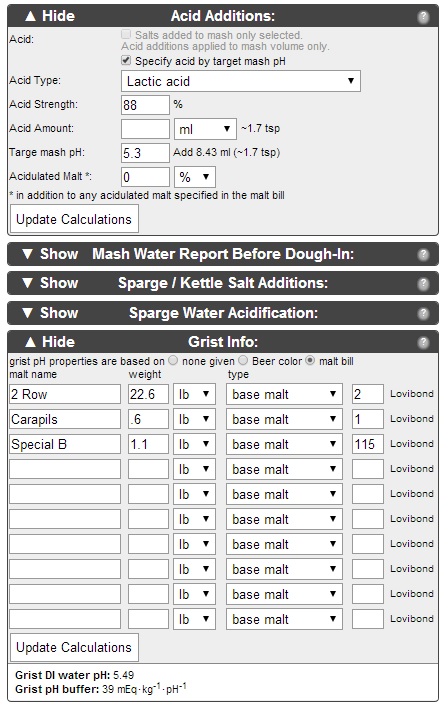
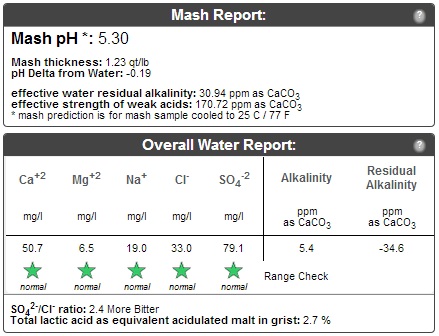
This is my second venture with adjusting my water profile to obtain proper mash pH and flavor profile. I was hoping some of you guys with more info could look this over and let me know if I'm on the right track.
I am planning to steep the Roasted Barley and Chocolate Malt in the boil kettle, to avoid them throwing off my mash pH. I've never done this before, since I've never calculated my pH before. I believe that is pretty common practice for this reason, right?
Thanks!




