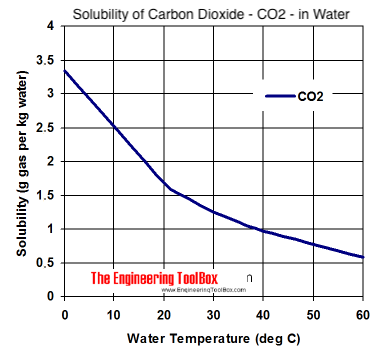
This weekend I'm trying to tap a 3 gal corny keg of a brew that spent 2 weeks in primary, then was racked to the keg with 6 Tbsp corn sugar simple syrup. It has been in keg for 2 weeks at 72F. The day it was kegged I charged it with a cylinder of co2, bled it, and recharged to evacuate oxygen.
I tapped today... Two things: 1. Tons of foam, 2. Very little carbonation.
I have put the keg into my fridge, in hopes that they reduced temp will help the beer absorb some more co2. There appears to be plenty of co2 pressure on the keg.
Any recommendations ? I plan on trying the keg again after 24 hrs.
Thanks !
Beerluvva
I tapped today... Two things: 1. Tons of foam, 2. Very little carbonation.
I have put the keg into my fridge, in hopes that they reduced temp will help the beer absorb some more co2. There appears to be plenty of co2 pressure on the keg.
Any recommendations ? I plan on trying the keg again after 24 hrs.
Thanks !
Beerluvva