You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Is it better to age before or after stabilizing?
- Thread starter NeverDie
- Start date

Help Support Homebrew Talk - Beer, Wine, Mead, & Cider Brewing Discussion Forum:
This site may earn a commission from merchant affiliate
links, including eBay, Amazon, and others.
RPh_Guy
Bringing Sour Back
After stabilizing. Stabilizing prevents unwanted microbial activity and oxidation during aging.
There's also theoretical risk of off-flavors if left on the lees for too long.
I've tried it both ways.
If you have off-flavors at the end of fermentation, that's another story. Yeast can remove diacetyl (buttery) and acetaldehyde (green apple), but it doesn't take long, a few days at the most.
There's also theoretical risk of off-flavors if left on the lees for too long.
I've tried it both ways.
If you have off-flavors at the end of fermentation, that's another story. Yeast can remove diacetyl (buttery) and acetaldehyde (green apple), but it doesn't take long, a few days at the most.
After stabilizing. Stabilizing prevents unwanted microbial activity and oxidation during aging.
There's also theoretical risk of off-flavors if left on the lees for too long.
I've tried it both ways.
If you have off-flavors at the end of fermentation, that's another story. Yeast can remove diacetyl (buttery) and acetaldehyde (green apple), but it doesn't take long, a few days at the most.
A few days when? After fermenting stops? The reason I ask is because most of my current ferments are finishing in 7 to 10 days, but I've been reading recommendations to keep in the primary for 1 month or so. Are you implying I can rack my BOMM style projects off the Lee's at 15 days without losing any "cleanup" benefits?
RPh_Guy
Bringing Sour Back
A healthy fermentation doesn't produce any off-flavors to be "cleaned up".
If it's clear and it tastes good a few days after finishing, go ahead and rack/stabilize.
If it doesn't taste good when it's done fermenting, that means something went wrong (probably with fermentation; mead is rather unforgiving). Try to figure out what it was and correct it in the next batch.
Also, leaving it in primary doesn't hurt, as long as you haven't introduced oxygen. The exception is if the yeast produced hydrogen sulfide during fermenting, then you should get it off the lees quickly.
Hope this makes sense.
If it's clear and it tastes good a few days after finishing, go ahead and rack/stabilize.
If it doesn't taste good when it's done fermenting, that means something went wrong (probably with fermentation; mead is rather unforgiving). Try to figure out what it was and correct it in the next batch.
Also, leaving it in primary doesn't hurt, as long as you haven't introduced oxygen. The exception is if the yeast produced hydrogen sulfide during fermenting, then you should get it off the lees quickly.
Hope this makes sense.
NeverDie
Well-Known Member
After stabilizing. Stabilizing prevents unwanted microbial activity and oxidation during aging.
There's also theoretical risk of off-flavors if left on the lees for too long.
I've tried it both ways.
If you have off-flavors at the end of fermentation, that's another story. Yeast can remove diacetyl (buttery) and acetaldehyde (green apple), but it doesn't take long, a few days at the most.
OK, so stabilize first and then age.
Time for my noob question then: exactly what is doing the aging during the aging process if the yeast are largely incapacitated? Is it simply very slow to happen chemical reactions, or is it the yeast that have somehow managed to survive the stabilizing process that go on to "age" the mead?
NeverDie
Well-Known Member
A few days when? After fermenting stops? The reason I ask is because most of my current ferments are finishing in 7 to 10 days, but I've been reading recommendations to keep in the primary for 1 month or so. Are you implying I can rack my BOMM style projects off the Lee's at 15 days without losing any "cleanup" benefits?
How many days after pitching does your mead go clear? I suspect the one month figure you're referring to is about that length of time, give or take.
RPh_Guy
Bringing Sour Back
From my limited understanding, both spontaneous reactions, particularly oxidation, and also yeast activity change the flavor compounds over time.exactly what is doing the aging during the aging process
Oxygen seeps in through the packaging, causing low amounts of oxidation over time.
Neither sorbate nor sulfite actually kill the yeast, so unless you sterile filter or pasteurize, the yeast (and bacteria) will still be present.
FYI: Different yeast strains have different effects during aging.
How many days after pitching does your mead go clear? I suspect the one month figure you're referring to is about that length of time, give or take.
Honestly it depends on the ambient temps. This time of year I would say 7 to 20 days depending on what else I have put in besides honey water and yeast. During the cooler months, significantly less.
Interestingly enough I have a sour cherry cider that finished up about a week ago with abaye yeast. It had dropped mostly clear in about 2 weeks. I racked it and topped off with sweet cider (which was loaded with sediment and clouded it up, but that all dropped over night) to keep the O2 to a minimum and was going to let it sit in secondary for a month or two but 2 days later it woke up and started eating the sugar in the new juice. Figuring I was at the upper limit of alcohol tolerance for the yeast I decided to bottle right away because I wanted it carbonated. This was last Saturday. The cider flavor was good but the tart cherry imparted a bit of a cough medicine flavor that worried me, but I had no choice but to continue. It is now Tuesday, I just returned from the airport and decided to give one a try. Room temp, gave me a bit of a geyser but the flavor is exquisite. Nothing like it was 2 days ago. That's the good news. The bad news is I better chill this stuff in a hurry because I suspect room temp aging is gonna make me sad with bottle bombs.
NeverDie
Well-Known Member
Honestly it depends on the ambient temps. This time of year I would say 7 to 20 days depending on what else I have put in besides honey water and yeast. During the cooler months, significantly less.
Interestingly enough I have a sour cherry cider that finished up about a week ago with abaye yeast. It had dropped mostly clear in about 2 weeks. I racked it and topped off with sweet cider (which was loaded with sediment and clouded it up, but that all dropped over night) to keep the O2 to a minimum and was going to let it sit in secondary for a month or two but 2 days later it woke up and started eating the sugar in the new juice. Figuring I was at the upper limit of alcohol tolerance for the yeast I decided to bottle right away because I wanted it carbonated. This was last Saturday. The cider flavor was good but the tart cherry imparted a bit of a cough medicine flavor that worried me, but I had no choice but to continue. It is now Tuesday, I just returned from the airport and decided to give one a try. Room temp, gave me a bit of a geyser but the flavor is exquisite. Nothing like it was 2 days ago. That's the good news. The bad news is I better chill this stuff in a hurry because I suspect room temp aging is gonna make me sad with bottle bombs.
Well, your examples aren't meads. That muddies the water with extraneous data.
Well, your examples aren't meads. That muddies the water with extraneous data.
Good point. Muddying the waters was the opposite of my intention.
Getting back on track, I'm reading that oxidation is less of a problem for mead than other wines. If this is true, would it be reasonable to assume that oxidation has less of an impact on the aging process for mead? And if that is a correct assumption, does this lend to mead inherently needing more time to properly age, or would it mean the yeast is the dominant force in the aging process?
OK, so stabilize first and then age.
At least add sulfite for an antioxidant before aging. I usually don't add sorbate until bottling time, and then only if back sweetening.
Time for my noob question then: exactly what is doing the aging during the aging process if the yeast are largely incapacitated? Is it simply very slow to happen chemical reactions, or is it the yeast that have somehow managed to survive the stabilizing process that go on to "age" the mead?
There are different levels of aging, some that involve the yeast and some that don't. I've never really looked into it but I do know that some people age on the "fine" lees, after secondary. A few cider guys say that leaving cider on the gross lees of D47 adds "complexity".
Then there's other stuff that has nothing to do with yeast. Why do the flavors in a JAOM meld and come together with time? Why do ciders get more appley with time? Why do fusels diminish with time, even in a closed bottle? I have no idea.
With wine, they say acids and tannins change with age. One could spend a lifetime studying this stuff (and I'm sure some have).
https://www.tyrrells.com.au/educati...h-scott-richardson-retail-events-coordinator/
NeverDie
Well-Known Member
It seems like stabilizing may (?) also help with clarifying the must. I had a couple of test batches that I cold crashed, with little to no visible improvement--still very cloudy. But after adding the stabilizing agents and continuing the cold crash, a lot settled to the bottom and they cleared up quite a bit overnight.
Is it better to cold crash first, as I did, or is it better to add the stabilizing agents and then cold crash? Or, does it make no difference? My theory was that the cold crash would weaken anything alive in the must, and the stabilizing agents would then be more effective if applied after the cold crash had been going for, say, 24 hours or longer.
Is it better to cold crash first, as I did, or is it better to add the stabilizing agents and then cold crash? Or, does it make no difference? My theory was that the cold crash would weaken anything alive in the must, and the stabilizing agents would then be more effective if applied after the cold crash had been going for, say, 24 hours or longer.
loveofrose
Well-Known Member
It is better to cold crash first, rack the clear portion and stabilize. It is the free sulfite that provides protection. Sulfite that has bound to objects (yeast, fruit bits, etc) provides no protection and reduces free sulfite. If too much free sulfite becomes bound, then you are no longer stabilized at all.
In your case, much free sulfite became bound thus neutralizing charges and caused clearing. Bentonite and other clearing agents operate the same way, but better. This is also indicative that you may not be as stabilized as you hoped.
It is important to mention that both pH and bindable particulates determines how much is necessary to fully stabilize the mead. This is why you hear about failed stabilization. Not enough sulfite was added to overcome binding and pH. Read here for more accurate details of the proper way to dose it: https://morewinemaking.com/products/sorbistat-potassium-sorbate.html and https://morewinemaking.com/articles/SO2_management
Should you stabilize? That depends on you. Stabilizing does maintain the fresh flavors and provides insurance from oxidation; however, the meads don’t age the same way. They both age, just differently. Stabilized mead has less oxidation and gets smoother. Non-stabilized mead enjoys some micro oxidation and ages more like traditional red wine. Which is better depends on the mead!
In your case, much free sulfite became bound thus neutralizing charges and caused clearing. Bentonite and other clearing agents operate the same way, but better. This is also indicative that you may not be as stabilized as you hoped.
It is important to mention that both pH and bindable particulates determines how much is necessary to fully stabilize the mead. This is why you hear about failed stabilization. Not enough sulfite was added to overcome binding and pH. Read here for more accurate details of the proper way to dose it: https://morewinemaking.com/products/sorbistat-potassium-sorbate.html and https://morewinemaking.com/articles/SO2_management
Should you stabilize? That depends on you. Stabilizing does maintain the fresh flavors and provides insurance from oxidation; however, the meads don’t age the same way. They both age, just differently. Stabilized mead has less oxidation and gets smoother. Non-stabilized mead enjoys some micro oxidation and ages more like traditional red wine. Which is better depends on the mead!
NeverDie
Well-Known Member
It is better to cold crash first, rack the clear portion and stabilize. It is the free sulfite that provides protection. Sulfite that has bound to objects (yeast, fruit bits, etc) provides no protection and reduces free sulfite. If too much free sulfite becomes bound, then you are no longer stabilized at all.
In your case, much free sulfite became bound thus neutralizing charges and caused clearing. Bentonite and other clearing agents operate the same way, but better. This is also indicative that you may not be as stabilized as you hoped.
It is important to mention that both pH and bindable particulates determines how much is necessary to fully stabilize the mead. This is why you hear about failed stabilization. Not enough sulfite was added to overcome binding and pH. Read here for more accurate details of the proper way to dose it: https://morewinemaking.com/products/sorbistat-potassium-sorbate.html and https://morewinemaking.com/articles/SO2_management
Should you stabilize? That depends on you. Stabilizing does maintain the fresh flavors and provides insurance from oxidation; however, the meads don’t age the same way. They both age, just differently. Stabilized mead has less oxidation and gets smoother. Non-stabilized mead enjoys some micro oxidation and ages more like traditional red wine. Which is better depends on the mead!
Aha! Makes sense. So, I assume therefore that adding bentonite after stabilization will likely wipe out even more of the sulfite? i.e. it is better to follow these steps:
1. cold crash
2. rack off the clearer portion
3. apply bentonite and wait for it to settle
4. rack off the clear portion.
5. only then, stabilize!
loveofrose
Well-Known Member
Most modern mead makers add bentonite upfront so that the fermentation encourages the bentonite to contact as much surface as possible. It also prevents the “clay” flavor that can occur in secondary additions.
NeverDie
Well-Known Member
It is better to cold crash first, rack the clear portion and stabilize. It is the free sulfite that provides protection. Sulfite that has bound to objects (yeast, fruit bits, etc) provides no protection and reduces free sulfite. If too much free sulfite becomes bound, then you are no longer stabilized at all.
In your case, much free sulfite became bound thus neutralizing charges and caused clearing. Bentonite and other clearing agents operate the same way, but better. This is also indicative that you may not be as stabilized as you hoped.
It is important to mention that both pH and bindable particulates determines how much is necessary to fully stabilize the mead. This is why you hear about failed stabilization. Not enough sulfite was added to overcome binding and pH. Read here for more accurate details of the proper way to dose it: https://morewinemaking.com/products/sorbistat-potassium-sorbate.html and https://morewinemaking.com/articles/SO2_management
Should you stabilize? That depends on you. Stabilizing does maintain the fresh flavors and provides insurance from oxidation; however, the meads don’t age the same way. They both age, just differently. Stabilized mead has less oxidation and gets smoother. Non-stabilized mead enjoys some micro oxidation and ages more like traditional red wine. Which is better depends on the mead!
The second link on SO2 management refered to a chart that, unfortunately, wasn't in the article. However, for the convenience of anyone reading this, I think I found a very similar chart to reference:
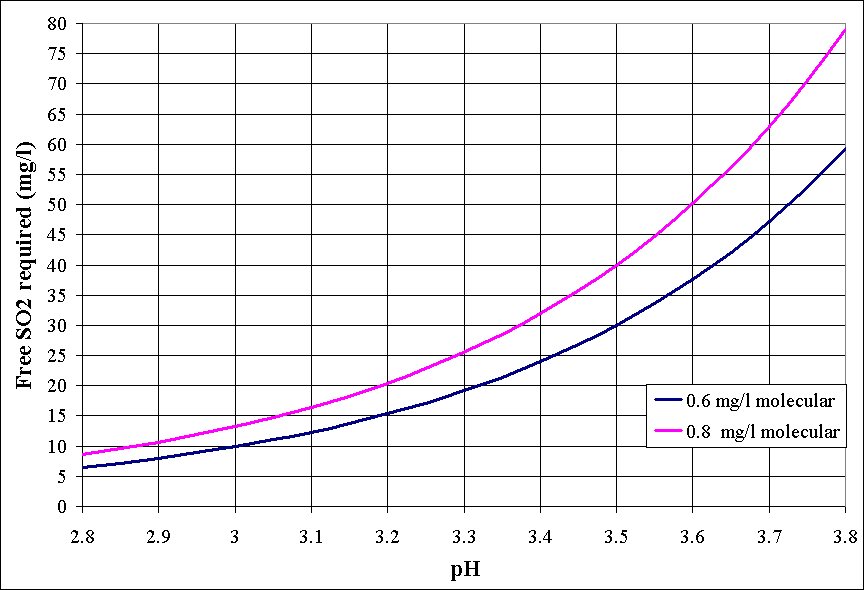
NeverDie
Well-Known Member
Which one of the three sulfite test kits did you buy?It is better to cold crash first, rack the clear portion and stabilize. It is the free sulfite that provides protection. Sulfite that has bound to objects (yeast, fruit bits, etc) provides no protection and reduces free sulfite. If too much free sulfite becomes bound, then you are no longer stabilized at all.
In your case, much free sulfite became bound thus neutralizing charges and caused clearing. Bentonite and other clearing agents operate the same way, but better. This is also indicative that you may not be as stabilized as you hoped.
It is important to mention that both pH and bindable particulates determines how much is necessary to fully stabilize the mead. This is why you hear about failed stabilization. Not enough sulfite was added to overcome binding and pH. Read here for more accurate details of the proper way to dose it: https://morewinemaking.com/products/sorbistat-potassium-sorbate.html and https://morewinemaking.com/articles/SO2_management
Should you stabilize? That depends on you. Stabilizing does maintain the fresh flavors and provides insurance from oxidation; however, the meads don’t age the same way. They both age, just differently. Stabilized mead has less oxidation and gets smoother. Non-stabilized mead enjoys some micro oxidation and ages more like traditional red wine. Which is better depends on the mead!
loveofrose
Well-Known Member
Which one of the three sulfite test kits did you buy?
I just put my own together like the video above.
I just don't see myself running a test that involved very often. I guess that means I would need the fully automated test kit.....
There are simpler ways to do that. Maybe not as accurate, but cheap enough -
https://morewinemaking.com/products/sulphite-test-kit.html
NeverDie
Well-Known Member
Unfortunately, it's not as easy to avoid as that, though. According to the second article that loveofrose linked to, you also need to compensate for any other kind of binding that might happen too.That's why I prefer not to bulk age after any kind of oxygen exposure. I don't want to monitor SO2 levels.
NeverDie
Well-Known Member
There are simpler ways to do that. Maybe not as accurate, but cheap enough -
https://morewinemaking.com/products/sulphite-test-kit.html
It's plus or minus 30ppm in red wine. What about traditional mead? Would it be more accurate in mead or, for some reason, less accurate?
NeverDie
Well-Known Member
There are simpler ways to do that. Maybe not as accurate, but cheap enough -
https://morewinemaking.com/products/sulphite-test-kit.html
Hmmm.... According to this:
ascorbic acid and/or tannins will throw off the results. Makes me wonder if opti-white would throw it off as well? If nothing else, I guess I would also need the fancier test kit just to answer questions like this.
It's plus or minus 30ppm in red wine. What about traditional mead? Would it be more accurate in mead or, for some reason, less accurate?
I haven't used my kit yet, but titration tests usually involve watching for a color change and I assume that's the issue with red wines. Same goes for testing TA, though with a pH meter you don't need the color indicator. Those automated SO2 testers are expensive.
NeverDie
Well-Known Member
I've tried adding bentonite upfront in two separate Hornindal test batches, and both times it went sulfurous about half-way through fermentation. I guess maybe because the Bentonite is sequestering away nutrients? For that reason, until I get that sorted, I'm resorting to adding Bentonite after fermentation completes.Most modern mead makers add bentonite upfront so that the fermentation encourages the bentonite to contact as much surface as possible.
Anyone else experience this?
I've tried adding bentonite upfront in two separate Hornindal test batches, and both times it went sulfurous about half-way through fermentation. I guess maybe because the Bentonite is sequestering away nutrients? For that reason, until I get that sorted, I'm resorting to adding Bentonite after fermentation completes.
Anyone else experience this?
Nope. When did you add it? I put it in a day after the first nutrient addition.
Edit: Bentonite can even be added after the 1/3 sugar break. It should have no affect on the nutrients.
NeverDie
Well-Known Member
Nope. When did you add it? I put it in a day after the first nutrient addition.
Edit: Bentonite can even be added after the 1/3 sugar break. It should have no affect on the nutrients.
I added it at the very beginning, before even pitching the yeast. I figured that way it would get the benefit of all the fermentation "boiling" that happens around high Krausen (which peaks around 1.09-1.095SG in these 1.105OG Hornindal test batches).
I'll try adding it later, as you suggest. Thanks!
NeverDie
Well-Known Member
Those automated SO2 testers are expensive.
One of them is around $300: https://www.thebeveragepeople.com/products/testing/vinmetrica-sc-100a-sulfite-tester.html
Well, I guess in-for-an-inch eventually becomes in-for-a-mile. Is this Vinmetrica the best that there is, or is there anything faster/easier or more automated than the SC100A?
RPh_Guy
Bringing Sour Back
I've generally used wine yeast, so I'm assuming the yeast produced some marginal amount of SO2 and used up all the binding sites.Unfortunately, it's not as easy to avoid as that, though. According to the second article that loveofrose linked to, you also need to compensate for any other kind of binding that might happen too.
I agree it's not the best, but it's been working for me so far (my ciders are retaining fresh fruit character). I'm not really focused on wine making and testing SO2 seems like a pain in the ass, so I'll only do that if I find that I can't prevent off-flavors/oxidation using a minimalist approach.
Just my philosophy.
- Joined
- Jun 2, 2008
- Messages
- 64,951
- Reaction score
- 16,517
One of them is around $300: https://www.thebeveragepeople.com/products/testing/vinmetrica-sc-100a-sulfite-tester.html
Well, I guess in-for-an-inch eventually becomes in-for-a-mile. Is this Vinmetrica the best that there is, or is there anything faster/easier or more automated than the SC100A?
If you're going to get that, might as well just get the 300 and be able to measure pH and TA as well as SO2...
One of them is around $300: https://www.thebeveragepeople.com/products/testing/vinmetrica-sc-100a-sulfite-tester.html
Well, I guess in-for-an-inch eventually becomes in-for-a-mile. Is this Vinmetrica the best that there is, or is there anything faster/easier or more automated than the SC100A?
I think that one is the industry standard. I haven't been able to justify it. I bought the titration kit and haven't even used that yet. I've been using the old "50 ppm" 1/4 tsp K-Meta or 1 Campden per gallon and winging it. So far nothing has grown in any of my brews and I haven't had geraniums in a bottle, so I guess it's working.
NeverDie
Well-Known Member
It occurs to me that an overly simplistic way (perhaps not the optimal way) to approach this would be from the get-go to simply add the maximum about of potassium metabisulfite that can't be tasted--which would be how much exactly? That might give enough headroom to cover a multitude of sins, and who really cares if it's more than the minimum needed? Apparently it's just the total amount of SO2 added that's what you taste, not the free SO2 per se.
This way, testing wouldn't matter or be needed, because you've already added the maximum amount possible without ruining the taste. Aside from possible allergies, is there any downside to this brute force approach?
This way, testing wouldn't matter or be needed, because you've already added the maximum amount possible without ruining the taste. Aside from possible allergies, is there any downside to this brute force approach?
- Joined
- Jun 2, 2008
- Messages
- 64,951
- Reaction score
- 16,517
Aside from possible allergies, is there any downside to this brute force approach?
Yes,
Free SO2 continues to be lost over time (dissipation and/or binding), so you don't know when, and how much, you need to add to optimize the needed amount.
As mentioned, you can estimate by using some of the old wine techniques (adding 50ppm at every other racking or every few months), but without accurate measurement, you'll never know if the free SO2 is at the optimal amount.
NeverDie
Well-Known Member
Off flavor occurs from molecular SO2 over ~ 1.0-1.2 ppm.
I don't know how to interpret that. How many grams/gallon of potassium metabisulfite does that translate into? Regardless, it would be nice to know the ceiling on the potassium metabisulfite additions.
Last edited:
NeverDie
Well-Known Member
Even the SC100A is a bit more involved than what I'd prefer. In contrast, it looks as though the Titrets are definitely the easiest and fastest way to test:
Maybe 30 seconds and then done. Virtually no setup or cleanup. I like that.
Just as the best camera is the one I have with me, the best SO2 test is the one that I'm willing to actually use.
For mead, has anyone compared the results of a Titrets with one of the more accurate methods? At least for a traditional mead that's clear amber, color won't be a problem.
Note: The guy in the video forgot to check for pH to determine how much SO2 he should have. Although he seems happy at 30ppm, if it's plus or minus 30ppm, he might well have zero! If 30ppm is, in fact, the right target amount, it seems to me that he should have added another 30ppm SO2 just to be sure.
Maybe 30 seconds and then done. Virtually no setup or cleanup. I like that.
Just as the best camera is the one I have with me, the best SO2 test is the one that I'm willing to actually use.
For mead, has anyone compared the results of a Titrets with one of the more accurate methods? At least for a traditional mead that's clear amber, color won't be a problem.
Note: The guy in the video forgot to check for pH to determine how much SO2 he should have. Although he seems happy at 30ppm, if it's plus or minus 30ppm, he might well have zero! If 30ppm is, in fact, the right target amount, it seems to me that he should have added another 30ppm SO2 just to be sure.
Last edited:
RPh_Guy
Bringing Sour Back
For how frequently sulfite is used, there's a surprising lack of information available about how to use it. I think that's why most of us just wing it.
I'll try to explain what I know.
First, consider that sulfite products have a shelf life and degrade over time, especially with more oxygen exposure, so you might not be adding as much as you think you're adding.
To complicate matters, also remember that yeast produce sulfite during fermentation, varying by strain and possibly other variables. For example some wine strains allegedly may finish with up to 30ppm of sulfite in the wine.
Then, when you add metabisulfite to a liquid it reacts with oxygen. 1ppm dissolved oxygen (DO) neutralizes (oxidizes to sulfate) approximately 5ppm of the metabisulfite. If you aerated the heck out of it, it may contain up to 8ppm DO, neutralizing about 40ppm of the metabisulfite pretty much immediately.
After that, some of the resulting sulfite binds to various organic compounds (e.g. aldehydes) in the wine/must. Exactly how much binds is impossible to predict; I see figures ranging from 30-70%. (Why that's expressed as a percentage doesn't seem to make sense given that there's a finite amount of binding sites, so I don't even trust those widely variable numbers to be accurate.)
I hypothesize that binding capacity in wine is negligible because of sulfite produced during fermentation, but I have no data to support that.
Any remaining (unbound) sulfite is considered "free SO2", which is supposedly what protects the wine from oxidation since it's available to react with oxygen.
The bound sulfite may also be helping prevent oxidation to some degree.
In acidic aqueous media (e.g. wine), sulfite exists in equilibrium with molecular SO2. The percentage of molecular SO2 depends on the pH. You posted the chart above, I use a sulfite calculator.
Molecular SO2 is responsible for anti-microbial activity and supposedly is also what can cause off-flavor/aroma in the concentration I mentioned. Burnt match.
Allegedly 0.4ppm molecular SO2 starts to significantly inhibit bacteria/wild yeast and 0.8ppm greatly inhibits or kills the wild microbes and can start to affect Sacc. Sulfite tolerance varies by species and strain.
To know how much metabisulfite can be safely added to stabilize, you need to either know or assume that none of it will bind, or pick an arbitrary amount. Then after measuring pH you can calculate how much metabisulfite is needed to hit the target molecular SO2.
Edit: Of course, you can measure the free sulfite to a variable degree of accuracy and then calculate how much of that is molecular SO2.
To further complicate matters, bottling and aging both introduced oxygen, which will lower the amount of free sulfite.
Also FYI potassium and sodium metabisulfite are not interchangable since they provide different levels of sulfite by weight.
Campden is designed to provide 50ppm total sulfite at a rate of 1 tab per imperial gallon, or about 65ppm for 1 tab per US gallon.
Welcome to the crazy mixed up world of sulfite.
I'll try to explain what I know.
First, consider that sulfite products have a shelf life and degrade over time, especially with more oxygen exposure, so you might not be adding as much as you think you're adding.
To complicate matters, also remember that yeast produce sulfite during fermentation, varying by strain and possibly other variables. For example some wine strains allegedly may finish with up to 30ppm of sulfite in the wine.
Then, when you add metabisulfite to a liquid it reacts with oxygen. 1ppm dissolved oxygen (DO) neutralizes (oxidizes to sulfate) approximately 5ppm of the metabisulfite. If you aerated the heck out of it, it may contain up to 8ppm DO, neutralizing about 40ppm of the metabisulfite pretty much immediately.
After that, some of the resulting sulfite binds to various organic compounds (e.g. aldehydes) in the wine/must. Exactly how much binds is impossible to predict; I see figures ranging from 30-70%. (Why that's expressed as a percentage doesn't seem to make sense given that there's a finite amount of binding sites, so I don't even trust those widely variable numbers to be accurate.)
I hypothesize that binding capacity in wine is negligible because of sulfite produced during fermentation, but I have no data to support that.
Any remaining (unbound) sulfite is considered "free SO2", which is supposedly what protects the wine from oxidation since it's available to react with oxygen.
The bound sulfite may also be helping prevent oxidation to some degree.
In acidic aqueous media (e.g. wine), sulfite exists in equilibrium with molecular SO2. The percentage of molecular SO2 depends on the pH. You posted the chart above, I use a sulfite calculator.
Molecular SO2 is responsible for anti-microbial activity and supposedly is also what can cause off-flavor/aroma in the concentration I mentioned. Burnt match.
Allegedly 0.4ppm molecular SO2 starts to significantly inhibit bacteria/wild yeast and 0.8ppm greatly inhibits or kills the wild microbes and can start to affect Sacc. Sulfite tolerance varies by species and strain.
To know how much metabisulfite can be safely added to stabilize, you need to either know or assume that none of it will bind, or pick an arbitrary amount. Then after measuring pH you can calculate how much metabisulfite is needed to hit the target molecular SO2.
Edit: Of course, you can measure the free sulfite to a variable degree of accuracy and then calculate how much of that is molecular SO2.
To further complicate matters, bottling and aging both introduced oxygen, which will lower the amount of free sulfite.
Also FYI potassium and sodium metabisulfite are not interchangable since they provide different levels of sulfite by weight.
Campden is designed to provide 50ppm total sulfite at a rate of 1 tab per imperial gallon, or about 65ppm for 1 tab per US gallon.
Welcome to the crazy mixed up world of sulfite.
Last edited:
Similar threads
- Replies
- 4
- Views
- 938

