Bigarcherynut
Well-Known Member
I'm hoping this is the correct forum to post this.
At the beginning of the year I finished brewing three batches in my BIAB E-System. This was my first attempt at all grain brewing and from some of the feedback was told to consider having my water tested.
All the batches were drinkable but one a Buffalo Sweat clone was ok but did not taste like the original at all. I looked at 3 different recipes and all were so very close I figured it should come out ok. Wondering if the actual out come was due to my water quality I decided before brewing any more batches would send in a sample of my water. I sent my sample to Ward Laborites for a Home Brewers test. I'm in the country and have a deep well. I talked to Ward labs and described my well and they feel the results would not vary during the seasons of the year so the one test result should be accurate.
I'm attaching the results of the test and hope you can give me some insight on what the results show and what I need to do to adjust my water for future brewing.
Thanks,
Bill
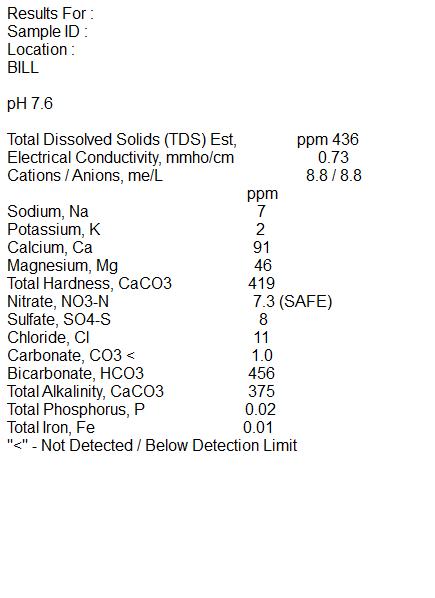
At the beginning of the year I finished brewing three batches in my BIAB E-System. This was my first attempt at all grain brewing and from some of the feedback was told to consider having my water tested.
All the batches were drinkable but one a Buffalo Sweat clone was ok but did not taste like the original at all. I looked at 3 different recipes and all were so very close I figured it should come out ok. Wondering if the actual out come was due to my water quality I decided before brewing any more batches would send in a sample of my water. I sent my sample to Ward Laborites for a Home Brewers test. I'm in the country and have a deep well. I talked to Ward labs and described my well and they feel the results would not vary during the seasons of the year so the one test result should be accurate.
I'm attaching the results of the test and hope you can give me some insight on what the results show and what I need to do to adjust my water for future brewing.
Thanks,
Bill




