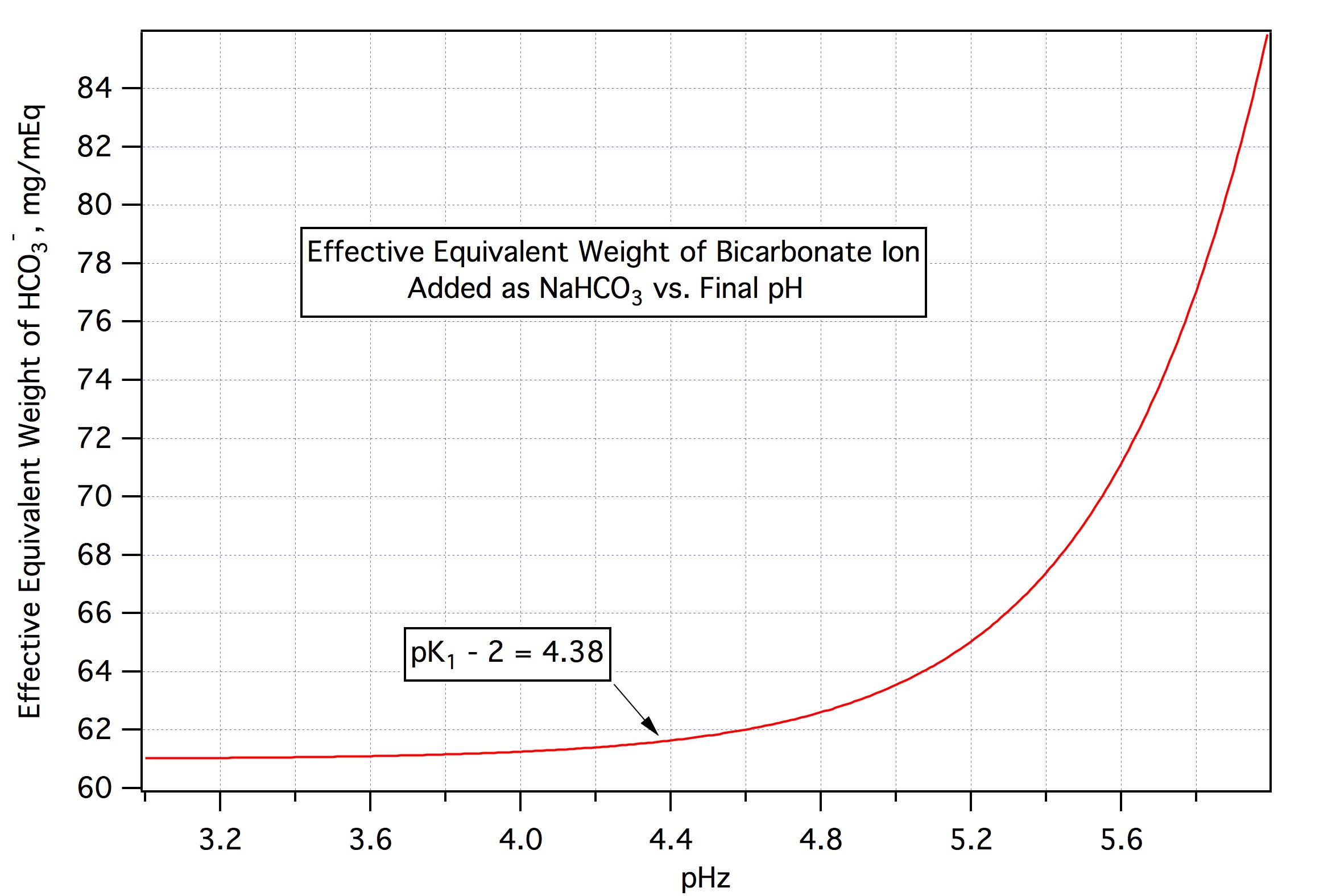
Sodium bicarbonate is often used to absorb protons where a grist contains a lot of high kilned (acidic) malts. The question addressed here is "How alkaline is bicarbonate from sodium bicarbonate?" (in other words, how much acid can it neutralize). Well the molecular weight of a bicarbonate ion is 61 mg/mmol and it carries a single negative charge so its equivalent weight must be 61 mEq/mg, no? No, unless the pH to which the mash is being adjusted is less than 4.38 (rule of thumb: pK - 2). Eighty-five mg (1 mmol containing 1 mmol of HCCO3-) of NaHCO3 does not absorb 1 mmol of protons above that pH. To absorb 1 mmol of protons requires the following amounts of bicarbonate ion depending on the target pH as shown:
pHz HCO3- required
5.3 66.6 mg
5.4 68.0
5.5 69.1
5.6 72.2
Thus these numbers represent the effective equivalent weight of the bicarbonate ion as a function of pHz. The sticky on carbonates and bicarbonates in this forum has the details on how to calculate these values.
So the reason for this post is that if you have created a spread sheet or calculator that assumes that each bicarbonate ion sucks up a proton at mash pH you will be in error. If you are using a spreadsheet that makes this assumption it is in error too. This same phenomenon occurs with acids (the normality of lactic and phosphoric acid depends on pHz) but the error incurred by assuming it constant is small because mash pH is pretty far from the closest acid pK. But with bicarbonate that distance is smaller (6.38 - 5.6 = 0.78; 6.38 - 5.3 = 1.08) and the error is, consequently larger.
I would assume that users of the popular spreadsheets will want reassurance that this is not a problem with the spreadsheets/calculators they use and I hope their authors will take a few minutes to check their programs and either correct them or let their users know that this is not a problem.
pHz HCO3- required
5.3 66.6 mg
5.4 68.0
5.5 69.1
5.6 72.2
Thus these numbers represent the effective equivalent weight of the bicarbonate ion as a function of pHz. The sticky on carbonates and bicarbonates in this forum has the details on how to calculate these values.
So the reason for this post is that if you have created a spread sheet or calculator that assumes that each bicarbonate ion sucks up a proton at mash pH you will be in error. If you are using a spreadsheet that makes this assumption it is in error too. This same phenomenon occurs with acids (the normality of lactic and phosphoric acid depends on pHz) but the error incurred by assuming it constant is small because mash pH is pretty far from the closest acid pK. But with bicarbonate that distance is smaller (6.38 - 5.6 = 0.78; 6.38 - 5.3 = 1.08) and the error is, consequently larger.
I would assume that users of the popular spreadsheets will want reassurance that this is not a problem with the spreadsheets/calculators they use and I hope their authors will take a few minutes to check their programs and either correct them or let their users know that this is not a problem.
Last edited: