Bigarcherynut
Well-Known Member
A while back I had my water tested by Ward Laboratories. I posted my results on the "All Grain" forum looking for help on what next to do. I was lead to a couple of different water calculators and ended up using Bru'n Water. I decided to post the results of my first attempt at correcting my water and was hoping for some feedback/input.
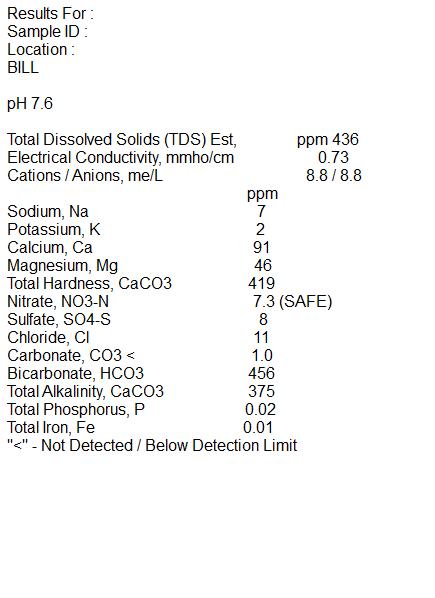
I have attached the results from Ward Labs.
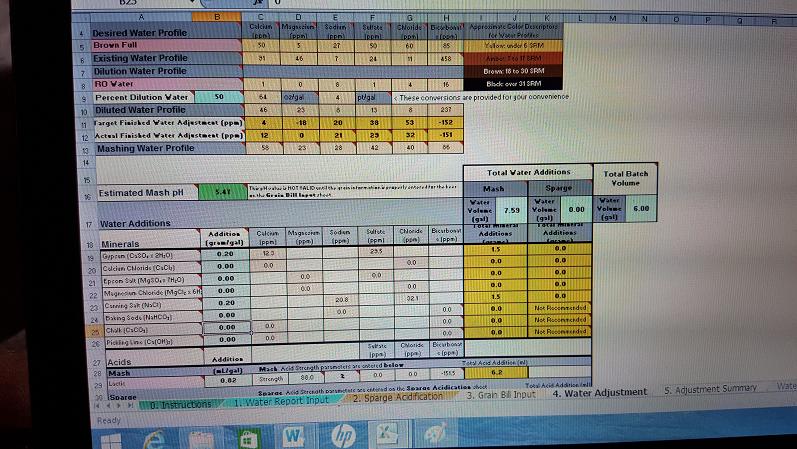
Here are my first corrections. I made the profile a Brown Full and the grain bill is for a Buffalo Sweat Cream Stout. The majority of beers I brew are porters and stouts so Im hoping this works for this style of beers.
Im using a 50 percent dilution with RO water due to my high hardness.
Im adding:
.2 grams per gal of Gypsum
.2 grams per gal of Canning salt
.82 ml per gal Lactic acid
Water Profile Calcium Mag Sodium Sulfate Chloride - Bicarbonate
Brown Full - 50 5 27 50 60 85
Existing Water 91 46 7 24 11 458
Mashing Profile 58 23 28 42 40 86
Estimated Mash Ph 5.47
Thanks for your input and advice.
I have attached the results from Ward Labs.
Here are my first corrections. I made the profile a Brown Full and the grain bill is for a Buffalo Sweat Cream Stout. The majority of beers I brew are porters and stouts so Im hoping this works for this style of beers.
Im using a 50 percent dilution with RO water due to my high hardness.
Im adding:
.2 grams per gal of Gypsum
.2 grams per gal of Canning salt
.82 ml per gal Lactic acid
Water Profile Calcium Mag Sodium Sulfate Chloride - Bicarbonate
Brown Full - 50 5 27 50 60 85
Existing Water 91 46 7 24 11 458
Mashing Profile 58 23 28 42 40 86
Estimated Mash Ph 5.47
Thanks for your input and advice.