Blazinlow86
Well-Known Member
- Joined
- Oct 19, 2016
- Messages
- 1,687
- Reaction score
- 743
curious about others experience with it? anyone confirm the ph tool works as well or better than brunwaters? which do you prefer or use? cheers
Last edited:

As stated on the other thread, valid feedback must clearly be objective and unbiased, and not tainted with confirmation bias, or given from the perspective of one who bears a chip on his shoulder.
Yes sir! As the creator of mashmadeeasy what would you descibe as improvements you have made over the other programs available? What's the best things about the software? Cheers
How does one find out the di_ph of there base malt? Does it still work without that info?It's ability to conform to the generally presumed initial (or default) DI_pH of your base malt by class, and beyond that, its ability to accept actually measured DI_pH's for all malts, including base malts.
That, plus it does not get mired in nasty water to grist ratio issues which can lead to serious errors with respect to the consistency of the softwares adjustment output advice as for some other of the currently popular choices of mash pH assistant software (which may be correct in output for some water to grist ratios, and incorrect in output for other water to grist ratios). This issue can be seen on the other thread.
How does one find out the di_ph of there base malt? Does it still work without that info?
Oh wow yea that's abit overly complicated for me. I don't need it down to the .001 just in the acceptable range. I'll give it a try using the defaults and compare to brunwater and report back. Seems to be a better comparison in real world practices. Cheers
Yup I have a decent meter and have been using it as long as brewing. No issues there. Another random question what made you decide on the name mashmadeeasy? Cheers
Any chance you would be willing to do a tutorial here to help new user's with all the extras required? Maybe just a simple recipe as a example?
I think it's a good idea. I think the reason it's not really catching on is it's overly complicated compared to brunwater. Have you ever done a side by side comparison between the 2? Is it that much more accurate the the easier brunwater method. I've always been within about 5% with brunwater. How much closer does mme get when doing all the extra stuff? Cheers
Silver- I use flaked rye quite often. Is there any appreciable difference in it's DI compared to oats? Same question for rice. The spec sheets are silent on these.
Cheers.
I'll sheepishly admit to having measured mash pH for the first time last week after 12 years of brewing.I have no personal experience with flaked rye, but D.M. Riffe has measured its DI_pH at a whopping 6.65. I remain somewhat suspect of this until it is verified by others. In your experience, has its use led to higher than predicted mash pH's?
I have no idea for rice. Sorry...
I'll sheepishly admit to having measured mash pH for the first time last week after 12 years of brewing.
I made a batch of saison that I didn't like at all and I'm narrowing down the issues. Mash water wasn't a problem, and the default for the rye is 6.2, so even overriding to 6.55 only moves the needle .02 for this recipe, which is coming in at 5.38. So I don't think mash pH was a problem either. It's taken a couple weeks of research to get to this point, but I design all of my recipes so now water chem and mash pH are in "the process".
If you're anywhere near Medina / Wadsworth, that's why you're seeing similar water.
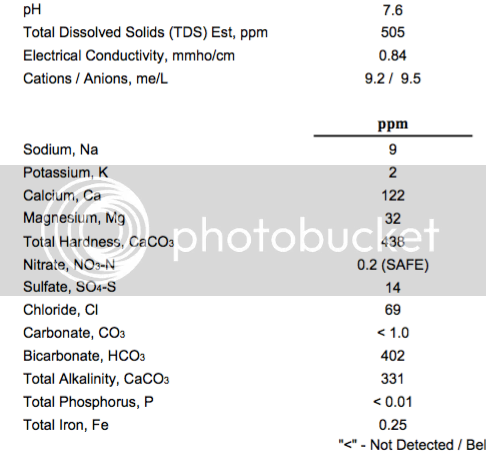
Just wanted to report in here /brag. I just sampled an english pale ale first time using 1469 yeasts, and also first time using the primer, MME and a pH meter. This batch mashed at 5.4 which was the projected target. I would up adding CaCl, gypsum and acid malt to the recipe as a result of the process. Simple grain bill 85% MO, 6% oats and rice, 3% acid malt. EKG's and Saaz to 27 IBU.
This is the clearest and cleanest beer I've ever done. It was that way going into the fermenter, and is that way coming out. Beer is excellent. Malt, yeast and hops and that's it. It's only 12 days old and I'll probably never know what it tastes like after 30 days because it will be gone by then.
I nailed the fermentation on this one, but I was impressed with how clean and clear the wort was going in. This is the first excellent beer I've made since moving to Ohio well water.
Enter your email address to join: