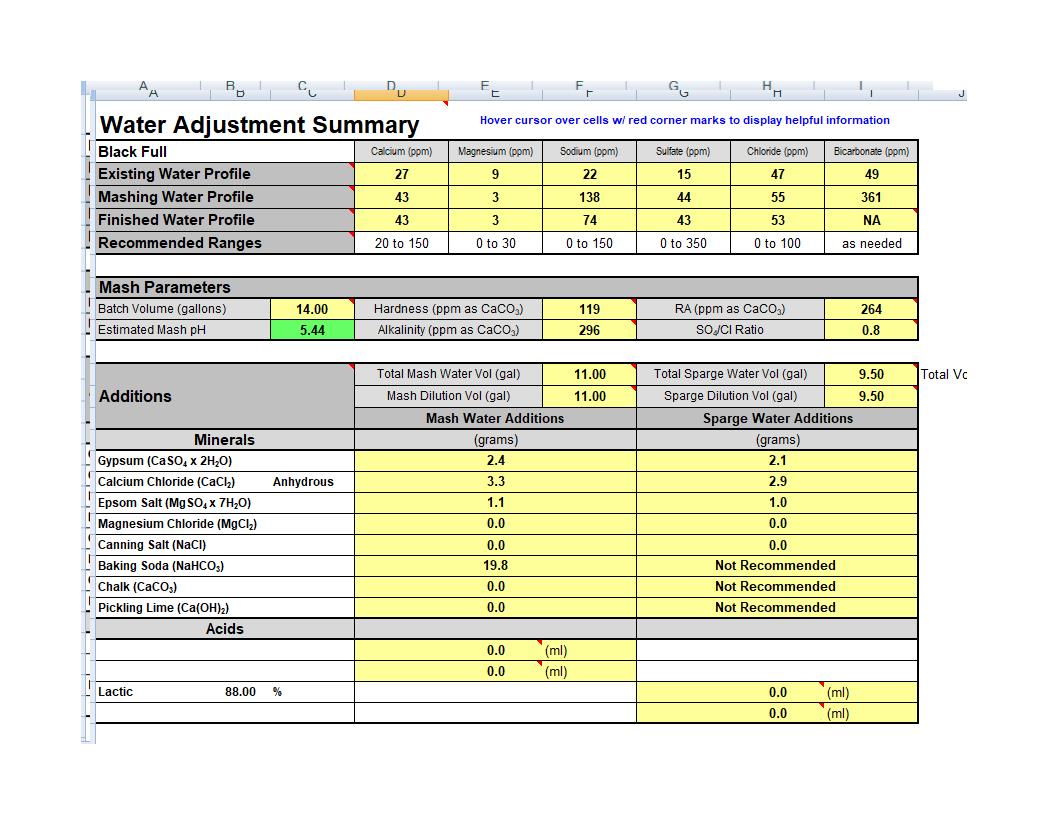
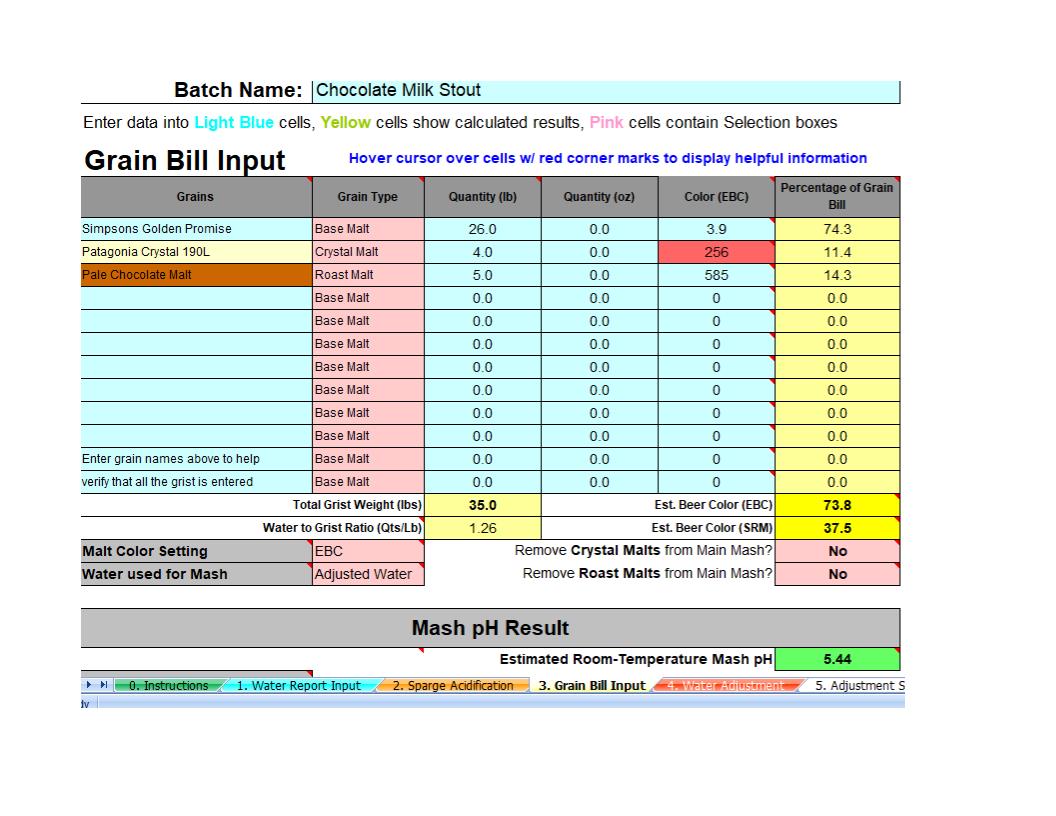
I am hoping I can get some feedback here. I've been using Bru'n water to build water profiles for my beers for a couple of years now and am somewhat comfortable using it.
I am making my first very dark beer (EBC 73.8) while building a water profile and am surprised by how acidic these dark malts make the mash. When I added the malts into the grain bill the unadjusted ph is predicted to be 4.3 and to get this up in the 5.4 range I have to add what seems to me to be a lot of baking soda, 1.8 grams per gallon. The overall finished water has only 74 ppm of Sodium which in my understanding is not too high and I was able to adjust the other minerals close to what the profile calls for (about 15-20% up or down depending on the mineral).
So, my question is: does this seem out of kilter with this baking soda addition?
I am making my first very dark beer (EBC 73.8) while building a water profile and am surprised by how acidic these dark malts make the mash. When I added the malts into the grain bill the unadjusted ph is predicted to be 4.3 and to get this up in the 5.4 range I have to add what seems to me to be a lot of baking soda, 1.8 grams per gallon. The overall finished water has only 74 ppm of Sodium which in my understanding is not too high and I was able to adjust the other minerals close to what the profile calls for (about 15-20% up or down depending on the mineral).
So, my question is: does this seem out of kilter with this baking soda addition?