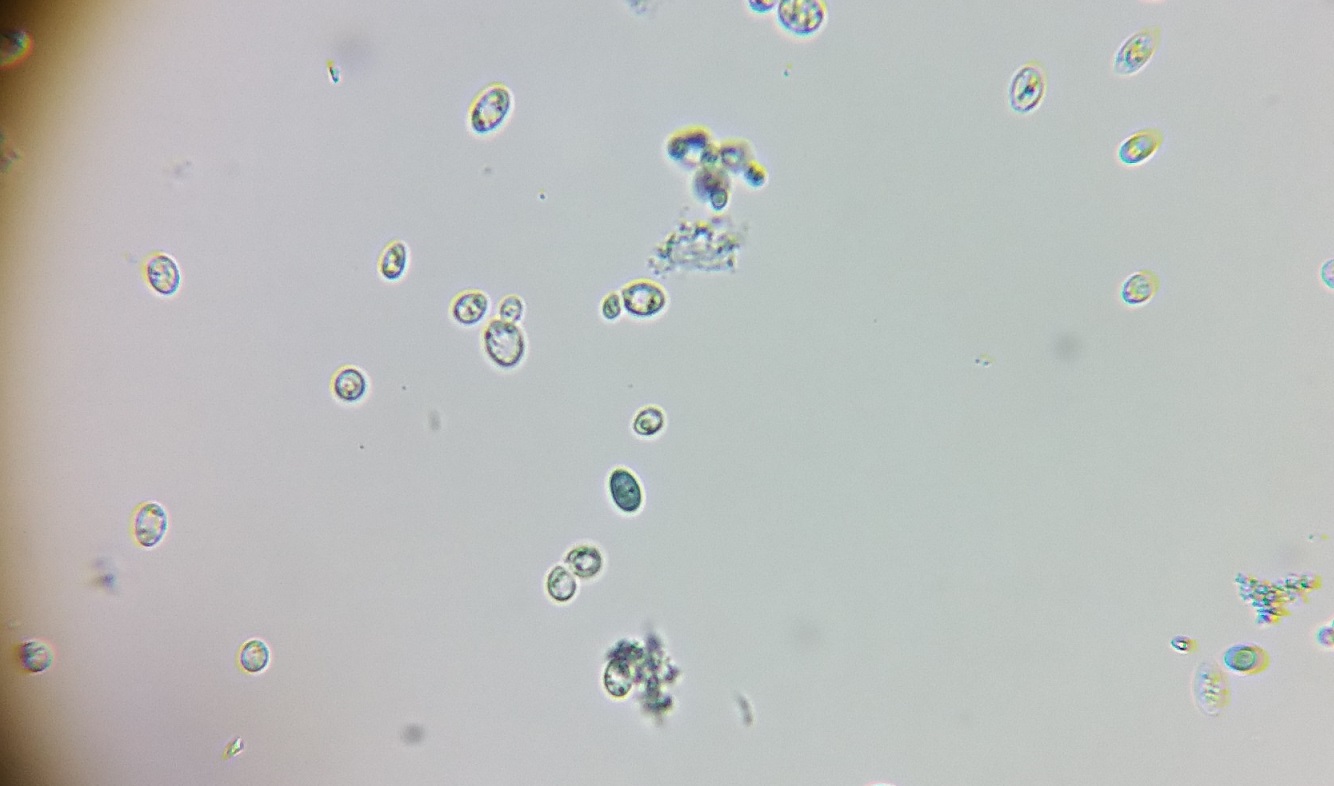
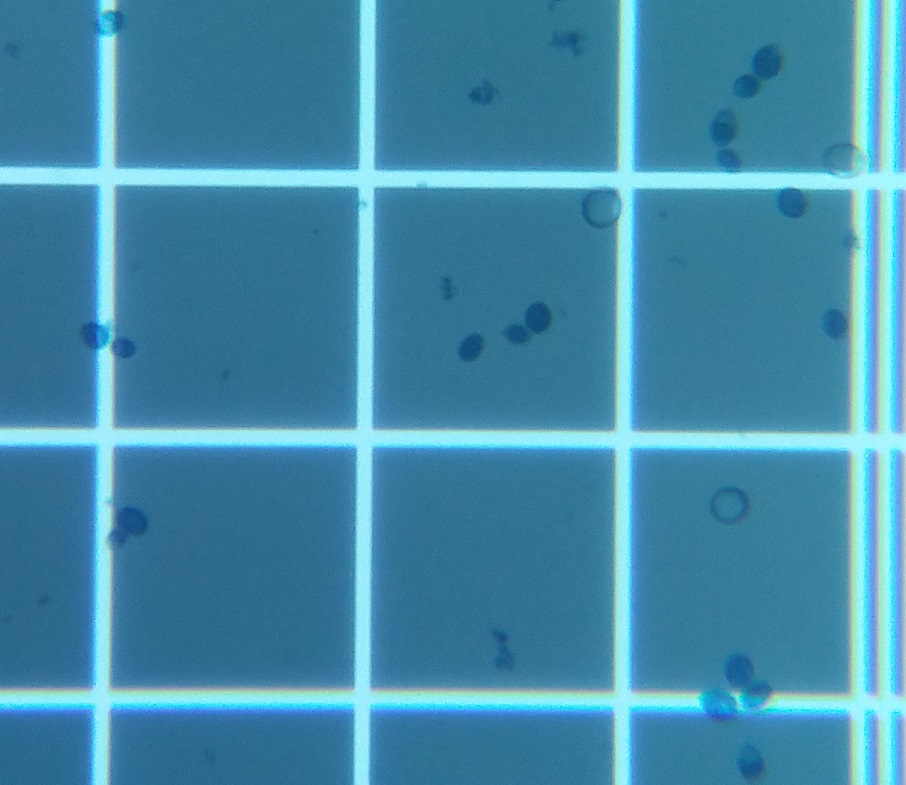
I attended a Yeast workshop put on by White Labs at the BREW boot camp in March 2019; one day was all the theory; the second day actual work in one of their labs which was very, very cool.
To figure out the number of cells in a starter (or a batch of brewing beer for that matter), you have to take a good sample and then dilute that sample several times using distilled water. You use specific tubes to do that (sort of like test tubes but plastic). I haven't done it in a while, but you sample a CC of beer or starter, add to the tube, add IIRC 9 CC of water, shake it up to mix thoroughly, sample that, and you do it three or four times (I don't recall exactly--don't have my notes handy).
Then you put the resulting solution under a microscope. We were told to use one that does 400x magnification, though if yours works with a hemocytometer, well, then it works. The cells sure look similar in size in your pic as they are in mine.
Then, you pick different squares in the hemocytometer and count yeast cells within those squares which is one heck of an easier way to do it.

And, anyone who does this professionally uses a counter/clicker instead of just saying "one, two, three...."
In a 5x5 grid of squares, for instance, you'd count the yeast in the four corner squares and the middle one, average the result, then use a multiplier to determine/estimate the actual number of cells, then multiply it back to determine the total number.
And as Vale71 says, you use ethylene blue to stain the cells. The live cells can keep the stain out; dead ones turn blue.
Below are two pics I took to show this. The first shows some yeast including a few that have turned blue. The second shows how the hemocytometer looks under the microscope. You can see the cells look like yours, except there are mostly blue ones. Kara, who taught the lab part of the workshop, nuked a sample in a microwave so we'd see what a mostly dead culture of yeast would look like. Most of the cells in the second pic are dead, but there are still a few hanging on.

Here are a couple more pics. The first shows yours truly peering into a microscope at White Labs. The second shows the kind of plastic tubes we're using.
Below are links to the tubes, microscope I have (which is what White Labs had

), clicker, and hemocytometer.
https://www.amazon.com/gp/product/B00UJGNU1G/ref=ppx_yo_dt_b_search_asin_title?ie=UTF8&psc=1
https://www.amazon.com/gp/product/B0094JTZOU/ref=ppx_yo_dt_b_search_asin_title?ie=UTF8&psc=1
https://www.amazon.com/gp/product/B071W3QTBX/ref=ppx_yo_dt_b_search_asin_title?ie=UTF8&psc=1
https://www.amazon.com/gp/product/B076ZT949V/ref=ppx_yo_dt_b_search_asin_title?ie=UTF8&psc=1