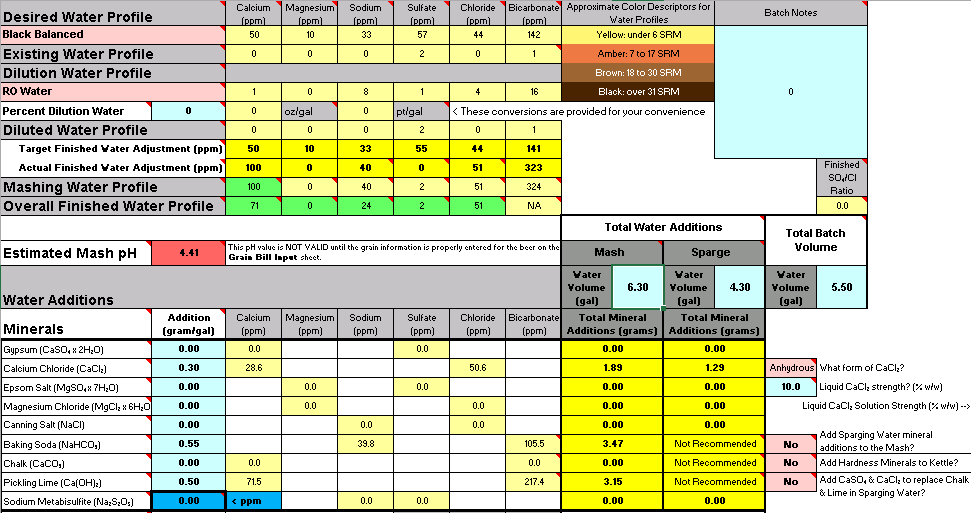
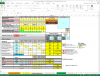
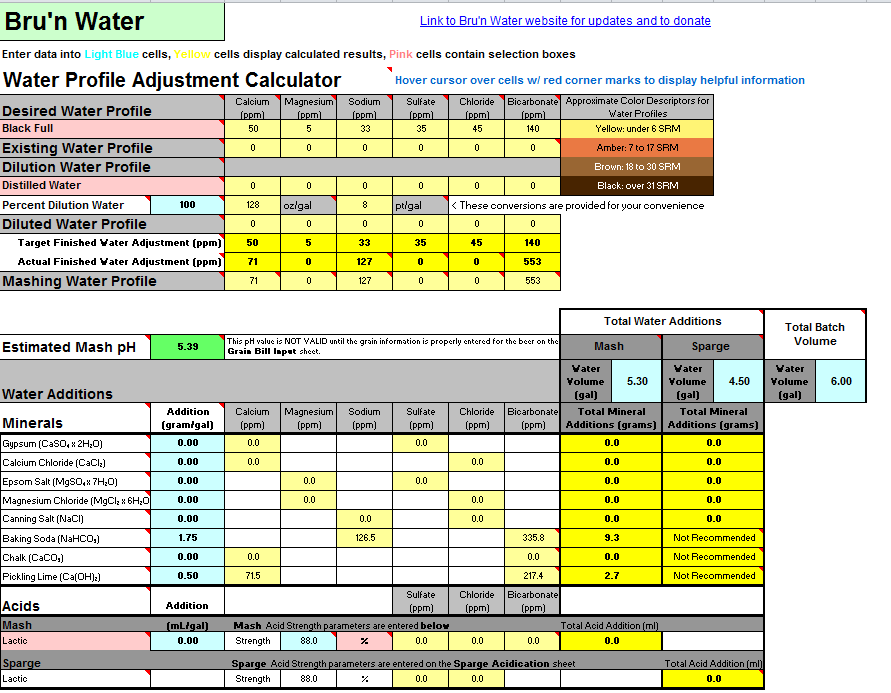
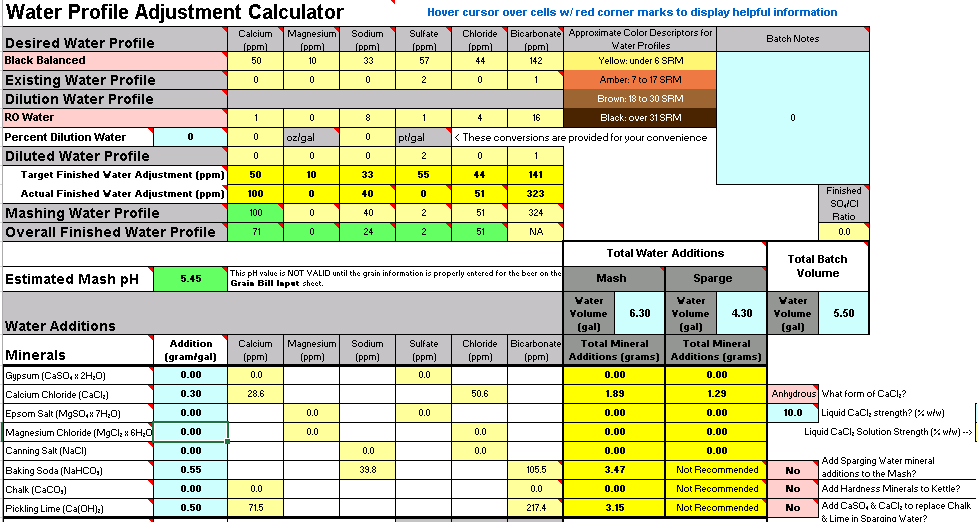
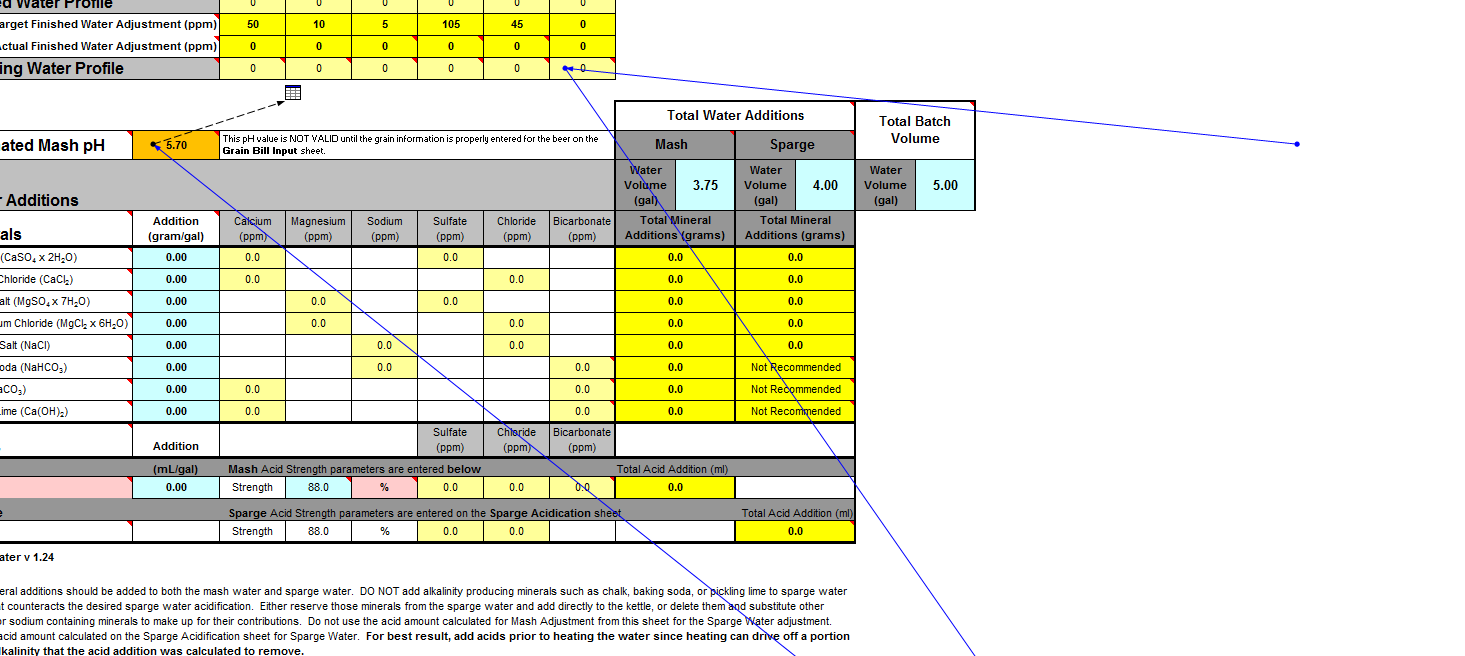
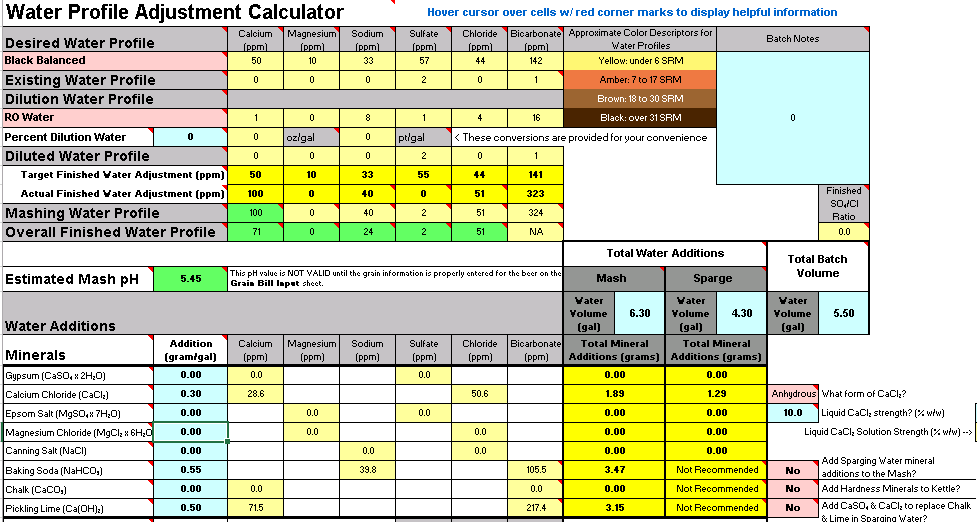
Working on an imperial stout recipe and I'm working on building the water profile in Bru'n Water. Given the grain bill, the mash pH is so low that it's taking an extreme amount of baking soda to bring it up, to the point that my Sodium PPM is through the roof at 217 and the bicarbonate level is at 576. I know bicarbonate isn't too critical, but the sodium is much higher than anticipated. (Disregard water volumes below, haven't changed those yet)
Do I change the grain bill to have less roast malts?
Is there something other than baking soda to bring up the mash pH to acceptable levels?


Do I change the grain bill to have less roast malts?
Is there something other than baking soda to bring up the mash pH to acceptable levels?