Echoloc8
Acolyte of Fermentalism
Brewed up a batch of Bee Cave IPA this past weekend, and in the interest of saving a few bucks I decided to grab some 5-gallon containers full of RO water from my local Walmart.
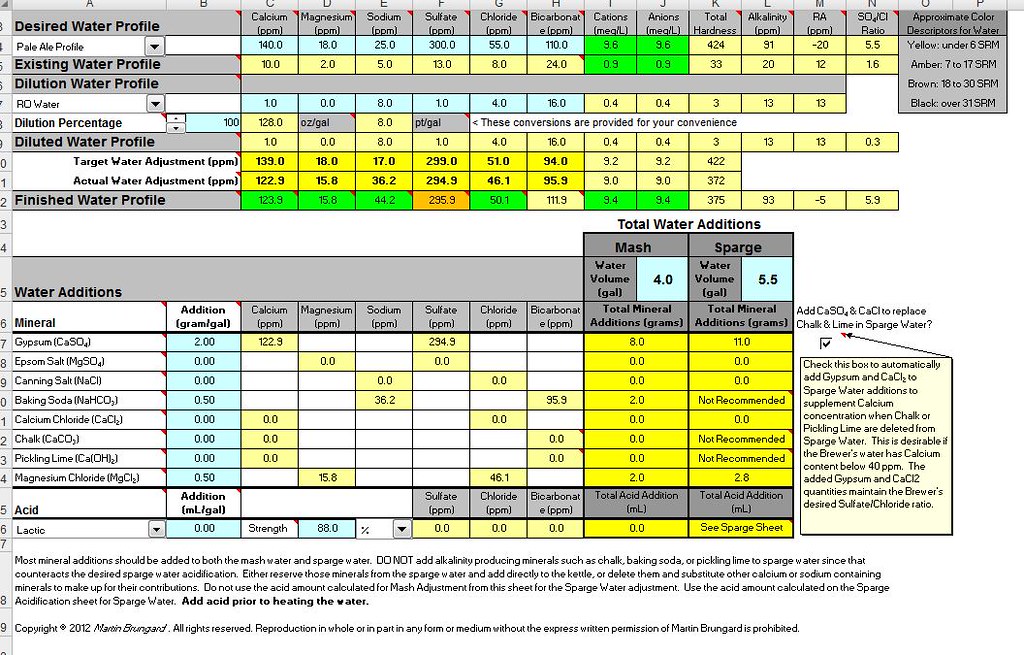
The water was indeed cheap, and I used the Bru'n Water site and spreadsheet to figure out my water adjustments right. I picked the Bru'n Water profile for Pale Ale, then found out I couldn't get Magnesium Chloride (MgCl2, one of its salt options that I'd chosen) on short notice.
So I used BeerSmith's water tool to recalculate amounts using the various other salts I did have available, and used those amounts in my mash and sparge water.
It all went really well! :rockin: I did have these questions:
-Rich
The water was indeed cheap, and I used the Bru'n Water site and spreadsheet to figure out my water adjustments right. I picked the Bru'n Water profile for Pale Ale, then found out I couldn't get Magnesium Chloride (MgCl2, one of its salt options that I'd chosen) on short notice.
So I used BeerSmith's water tool to recalculate amounts using the various other salts I did have available, and used those amounts in my mash and sparge water.
It all went really well! :rockin: I did have these questions:
- The water was very cloudy before dough-in and sparging, which I attribute to the Calcium Carbonate (chalk), just as a guess. Is this normal? None of the cloudiness transferred to the wort, which cleared beautifully while chilling and with Whirlfloc.
- My mash efficiency and brewhouse efficiency were *way* up from normal. Mash eff was something like 98% (normally 93-95%), while brewhouse was 75% on a beer I expected to get more like 65% on my system. Can additional minerals account for that, perhaps make the enzymes work better somehow?
-Rich